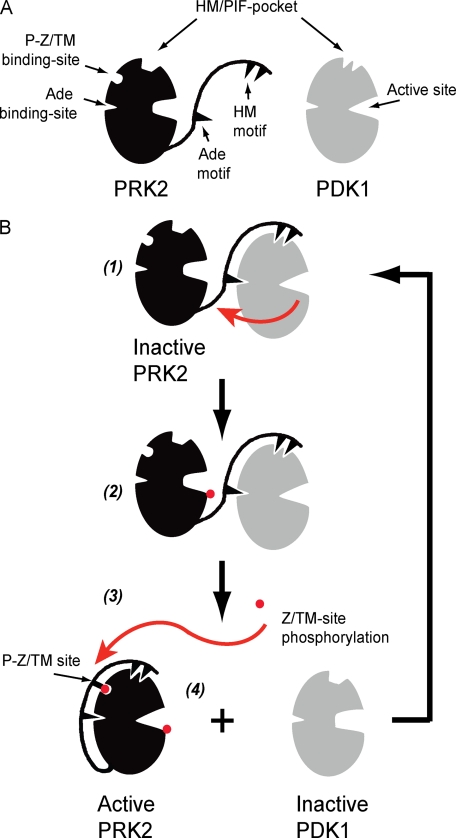
FIGURE 6.
Model describing the regulation of the interaction between PRK2 and PDK1. A, schematic representation of PRK2 and PDK1 showing the catalytic core possessing the HM/PIF-pocket, the phospho-Z/turn-motif binding site (P-Z/TM binding-site), the Ade binding site and the active site. The Ade and HM motifs are indicated within the C-terminal extension to the PRK2 catalytic core. The catalytic core of PRK2 and PDK1 are shown in inactive and active conformations, respectively. B, CT of inactive PRK2 is free to interact with PDK1; the interaction at the HM/PIF pocket of PDK1 brings PRK2 in close proximity to PDK1 and also prompts allosteric conformational changes in PDK1 that affect the ATP binding site and activates PDK1 to phosphorylate the substrate, PRK2. The interaction between PRK2 Ade motif and PDK1 Ade binding site is hypothetical (1 and 2). Phosphorylation of the Z/TM phosphorylation site (P-Z/TM site) triggers the interaction between this phosphate and the P-Z/TM binding site, acting like a zipper enabling the interaction of the HM and Ade motif to the PRK2 catalytic core (3 and 4). The increased interaction of the PRK2 CT with its own catalytic core prompts the dissociation from PDK1. In the absence of a bound HM, the PDK1 structure is stabilized in an inactive conformation, being again available for interaction with its substrates.