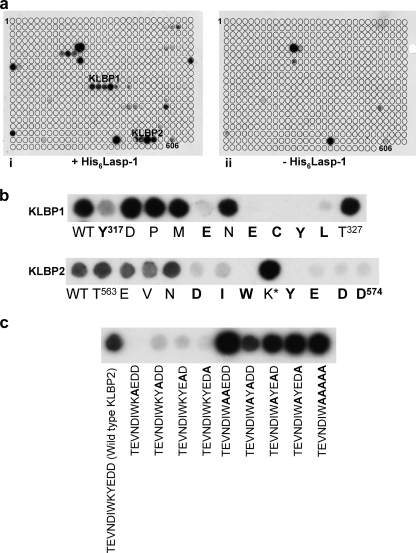
FIGURE 1.
Probing oligopeptide arrays revealed Lasp-1 binding motifs in Krp1. a, oligopeptide membranes that were arrays of decamer oligopeptides spanning the entire length of Krp1, modulating by +1 amino acid at a time, were probed with recombinant His6-Lasp-1. Two binding sites in the region of the C-terminal domain were identified (panel i), and these oligopeptides did not give a positive result on control membranes where no His6-Lasp-1 had been applied (panel ii). These two motifs corresponded to Tyr317–Thr327 (KLBP1) and Thr563–Asp574 (KLBP2). b, each motif was scanned by mutating each residue in turn to alanine. This demonstrated that the residues involved in Lasp-1 binding were Tyr317, Glu321, Glu323, Cys324, Tyr325, and Leu326 in KLBP1 and Asp567, Ile568, Trp569, Tyr571, Glu572, Asp573, and Asp574 in KLBP2. Mutation of Lys570 to alanine in KLBP2 consistently gave an increase in signal from Lasp-1 binding in replicate blots. WT, wild type. c, mutation of any of the 4 residues following Lys570 in KLBP2 resulted in an almost complete loss of Lasp-1 binding (all mutations are noted in bold). However, any or all of residues 571–574 could be removed without effect if Lys570 was mutated to alanine.