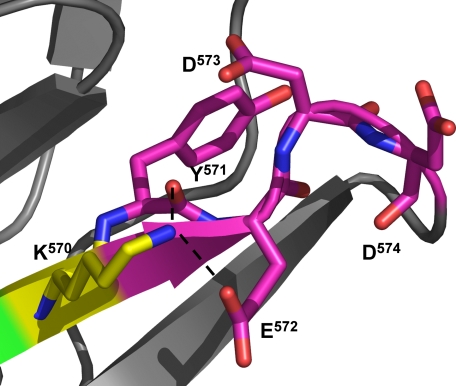
FIGURE 7.
Residues 571–574 reduce the inhibition of Lasp-1 binding to the Asp567–Ile568–Trp569 motif by Lys570. In the crystal structure, the side chain of Lys570 is exposed to the solvent but is surprisingly well ordered (averaged B-factor is 27.40 Å2 for Lys570, 28.96 Å2 for the Krp1 molecule). It appears to be held in place by a number of interactions including hydrogen bonds with a carboxyl oxygen of the Glu572 side chain and the main chain carbonyl oxygen of Tyr571. The positioning of residues 571–574 seems to be critical in a mechanism that tethers the Lys570 side chain in position.