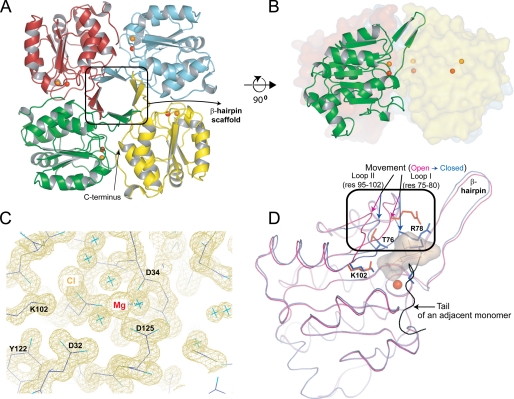
FIGURE 4.
A, structure of the KdsC tetramer showing the relative disposition of the β-hairpins responsible for tetramerization. The Mg2+ (red) and the Cl− (orange) located in the catalytic cleft are shown as spheres. B, the side view of the tetramer with one monomer highlighted. C, the 1.4 Å |2Fo − Fc| electron density map of the active site contoured at 1σ. D, conformational differences between the open (magenta) and the closed (blue) conformations of the active site. The Kdo8P binding site is highlighted with a shadow. The tail of an adjacent monomer is colored black. res, residues.