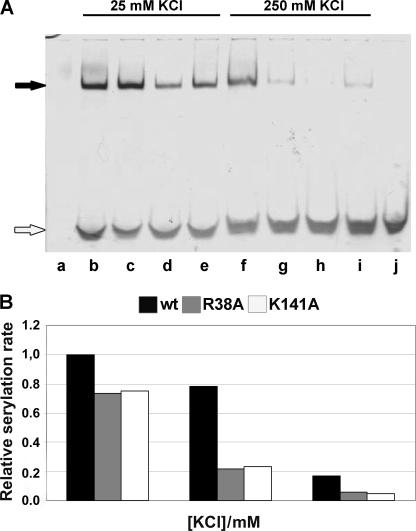
FIGURE 4.
Effect of different salt concentrations on non-covalent complex formation and serylation propensity. A, non-covalent complexes were made as described under “Experimental Procedures” and in the legend to Fig. 3, except that in the reaction mixture the concentration of KCl was varied as indicated, and then subjected to PAGE under native conditions. Non-complexed WT mMbSerRS is visible in lane a, whereas non-covalent complexes between tRNA and mMbSerRS variants in the presence of 25 mm KCl were loaded into following lanes: WT, lane b; R38A, lane c; K141A, lane d; and R143A, lane e; non-covalent complexes between tRNA and mMbSerRS variants in the presence of 250 mm KCl were loaded into following lanes: WT, lane f; R38A, lane g; K141A, lane h; and R143A, lane i; non-complexed tRNA is in lane j. Non-covalent complexes and non-complexed tRNA are marked with black and white arrows, respectively. B, relative serylation rate of the enzymes was tested at three different KCl concentrations (25, 125, and 250 mm). Activity of the WT enzyme at 25 mm KCl was taken as the reference point (100% activity). Contrary to the WT enzyme, a significant drop in the serylation rate was detected for both mutants after raising the KCl concentration from 25 to 125 mm.