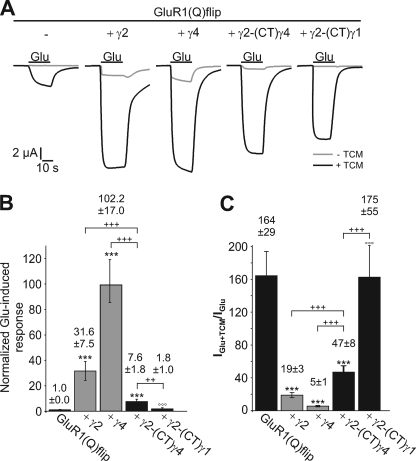
FIGURE 5.
Influence of γ2 carrying the C-terminal domain of γ4 on the extent of desensitization of GluR1(Q)flip. A, typical current responses of GluR1(Q)flip elicited by the application of 300 μm glutamate (Glu) in Xenopus oocytes in the absence (gray traces) and presence (black traces) of 600 μm TCM; analyzed were the receptor alone and in coexpression with γ2, γ4, and the two chimeras γ2-(CT)γ1 and γ2-(CT)γ4. Duration of Glu application is indicated by black bars. B, normalized glutamate-induced responses of GluR1(Q)flip in coexpression with γ2, γ4, and the two chimeras γ2-(CT)γ1 and γ2-(CT)γ4 (±S.E.). C, IGlu+TCM/IGlu ratios for GluR1(Q)flip alone and in coexpression with γ2, γ4, γ2-(CT)γ1, and γ2-(CT)γ4 were calculated for each oocyte and averaged (±S.E.). Black asterisks show significant differences compared with GluR1(Q)flip; open circles show differences of TARP chimeras compared with respective wild-type TARPs; plus symbols show differences of γ2-(CT)γ4 to γ2 and γ4 (++, p < 0.01; +++, ***, and °°°, p < 0.005; Student's t test; n = 5–14).