FIGURE 9.
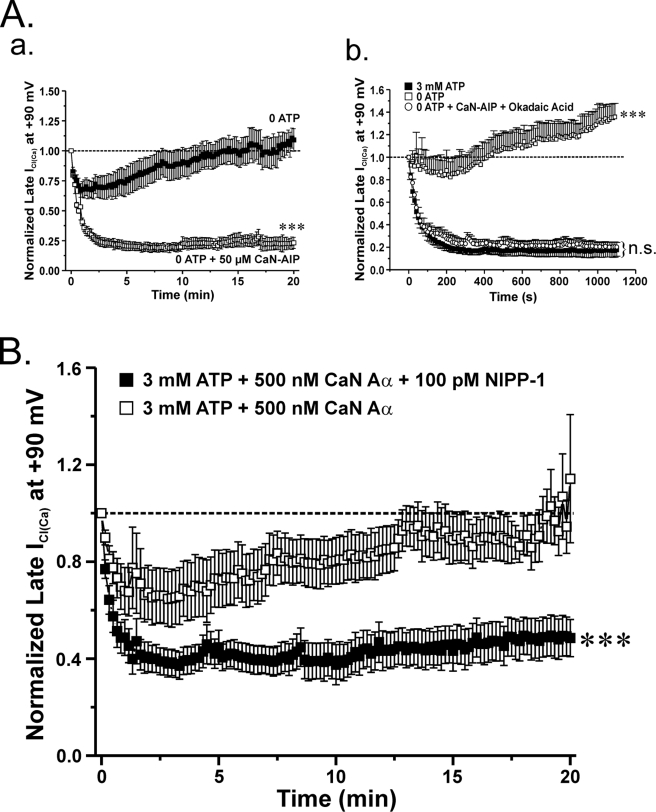
A functional link between calcineurin and PP1 appears to exist when it comes to the regulation of ICl(Ca) in rabbit pulmonary artery smooth muscle cells. A (a), mean time course of changes of normalized ICl(Ca) amplitude in the presence (open squares; n = 4) or absence (closed squares; n = 5) of 50 μm calcineurin autoinhibitory peptide (CaN-AIP). Exposure to 50 μm CaN-AIP suppressed the recovery of ICl(Ca). ClCa currents were reduced by ∼80% after 20 min when compared with control conditions. ***, significantly different from control (no CaN-AIP) with p < 0.001. A (b), graph showing the mean time course of changes of normalized ICl(Ca) at +90 mV (1-s steps at 10-s intervals) in cells dialyzed with 3 mm ATP (filled squares; n = 5) or no ATP, with (empty circles; n = 4) or without (empty squares; n = 5) 10 nm OA (PP1/PP2A inhibitor) and 50 μm CaN-AIP (CaN inhibitor). A one-way ANOVA test at the end of 20 min revealed that the population means were significantly different with p < 0.001 (***). n.s., not significant. B, to determine whether a functional link exists between CaN and PP1, our group examined the effects of the constitutively active calcineurin isoform CaN Aα in the presence of the PP1-specific inhibitor NIPP-1 under conditions that support phosphorylation. As indicated by the graph, which represents normalized late ICl(Ca) at +90 mV plotted as a function of time, 500 nm CaN Aα fully reverses the phosphorylation-mediated rundown of ICl(Ca) (open squares; n = 7). On the contrary, the effect of CaN Aα on ICl(Ca) recovery was significantly attenuated with the addition of 100 pm NIPP-1 to the pipette solution (closed squares; n = 10). The degree of rundown produced by 100 pm NIPP-1 was similar to that seen with 3 mm ATP. ***, significantly different from 3 mm ATP + 500 nm CaN Aα with p < 0.001.