Abstract
A single nucleotide polymorphism in Atg16L1, an autophagy-related gene (ATG), is a risk factor for Crohn disease, a major form of chronic inflammatory bowel disease. However, it is still unknown how the Atg16L1 variant contributes to disease development. The Atg16L1 protein possesses a C-terminal WD repeat domain whose function is entirely unknown, and the Crohn disease-associated mutation (T300A) is within this domain. To elucidate the function of the WD repeat domain, we established an experimental system in which a WD repeat domain mutant of Atg16L1 is stably expressed in Atg16L1-deficient mouse embryonic fibroblasts. Using the system, we show that the Atg16L1 complex forms a dimeric complex and that the total Atg16L1 protein level is strictly maintained, possibly by the ubiquitin proteasome system. Furthermore, we show that an Atg16L1 WD repeat domain deletion and the T300A mutant have little impact on canonical autophagy and autophagy against Salmonella enterica serovar Typhimurium. Therefore, we propose that Atg16L1 T300A is differentially involved in Crohn disease and canonical autophagy.
Introduction
Macroautophagy, referred to here as autophagy, is an intracellular process in which the cytosol and organelles are sequestered within double membrane-bound structures, called autophagosomes, which deliver their contents to the lysosome/vacuole for degradation (1). In addition to its physiological role in recycling intracellular materials as a starvation response, there is growing evidence that autophagy plays a role in many diverse biological phenomena, such as cellular immunity (2, 3).
The Atg16L1 protein forms a complex with the Atg12-Atg5 conjugate (referred to as the Atg16L1 complex) (4), and functions as an E32-like factor in LC3 lipidation (5, 6). The Atg16L1 protein consists of an N-terminal domain involved in Atg5 binding, a central coiled-coil domain required for self-oligomerization, and a C-terminal WD repeat domain whose role is entirely unknown (4). Recent genome-wide association studies have linked autophagy with Crohn disease, a major form of chronic inflammatory bowel disease (7–9). A single nucleotide polymorphism in Atg16L1 (T300A) is a risk factor for Crohn disease and resides in the WD repeat domain (7–9). However, it is still unknown how the Atg16L1 variant contributes to the development of Crohn disease.
Crohn disease is one form of idiopathic inflammatory bowel disease and develops predominantly at anatomical sites where commensal bacteria are dramatically increased in mass (10). Therefore, it is thought that bacterial flora has a crucial impact on the onset of Crohn disease, although this relationship is not well understood. The latest genome-wide association breakthroughs have expanded the role of innate immunity components beyond the already implicated Nod2 (11) to include autophagy based on the association of Atg16L1 with an autophagy-linked factor, immunity-related GTPase M (12). These associations suggest that the Atg16L1 T300A mutant may alter the antibacterial machinery that incorporates Atg16L1. A study using Atg16L1 knockdown cells and transient expression of small interference-resistant Atg16L1 constructs reported that the T300A mutant is fully functional in canonical autophagy but is less able to recruit LC3 to invading Salmonella enterica serovar Typhimurium (S. typhimurium) (13). However, because only a very small amount of the Atg16L1 complex is sufficient for full autophagic activity, residual levels of Atg16L1 may affect these interpretations (14). Furthermore, transient expression of Atg16L1 severely inhibits autophagy (5). Therefore, we decided to establish an experimental system that enables us to reliably analyze the function of the Atg16L1 WD repeat domain.
We have previously generated Atg16L1-deficient mice and demonstrated that Atg16L1 is indispensible for autophagosome formation (15). Therefore, Atg16L1-deficient cells should enable us to dissect the function of various exogenously expressed Atg16L1 mutants, such as a point mutant or deletion construct. In this report, we used an Atg16L1 reconstitution system to examine the biochemical nature of the Atg16L1 complex and analyze the function of the WD repeat domain that contains the Crohn disease-associated mutation (T300A).
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Cell culture reagents were purchased from Invitrogen. The following antibodies were used: anti-human Atg5 (16), anti-mouse Atg16L1 (4), anti-p62 (BIOMOL), anti-LC3 (Medical and Biological Laboratories), and anti-α-tubulin (clone B5-1-2, Sigma). All other reagents were purchased from Sigma-Aldrich.
DNA Engineering and Recombinant Retroviruses
pMRX-IRES-puro was a kind gift from Dr. S. Yamaoka (Tokyo Medical and Dental University, Japan) (17). To generate recombinant retroviruses, cDNAs corresponding to human Atg16L1β (isoform-1) constructs, including full-length (1–607), ΔWD repeat domain (1–249), and the Crohn disease-associated mutant (T300A), were subcloned into the pMRX-IRES-puro vector. Recombinant retroviruses were prepared as described previously (17).
Cell Culture, Plasmid Transfections, and Retrovirus Infections
Plat-E cells were generously provided by Dr. T. Kitamura (The University of Tokyo) (18). Wild-type, Atg5-deficient, and Atg16L1-deficient MEFs were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mm l-glutamine, and the appropriate antibiotics in a 5% CO2 incubator at 37 °C. For nutrient starvation, cells were cultured in Earle's Balanced Salt Solution (EBSS, Invitrogen) for 1 h. Transient transfections were carried out using Lipofectamine 2000 reagent (Invitrogen) following the manufacturer's protocol. Stable transformants were selected in growth medium with 1 μg/ml puromycin.
Western Blotting
Cells were washed with ice-cold phosphate-buffered saline, scraped, and collected by centrifugation at 4 °C. Cells were lysed in phosphate-buffered saline containing 2% Triton X-100, 1 mm phenylmethylsulfonyl fluoride, and a protease inhibitor mixture (Roche Applied Science) (19). Cell lysates were centrifuged at 15,000 × g for 15 min at 4 °C, and supernatants were collected. Samples were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membranes were blocked with 1% skim milk in 0.1% Tween 20/Trix-buffered saline and incubated with primary antibodies. Immunoreactive bands were detected using horseradish peroxidase-conjugated secondary antibodies (Jackson Laboratories) and luminol solution (1.25 mm luminol, 65 mm Tris-HCl (pH 8.0), 0.2 mm coumaric acid, 0.01% H2O2).
Fluorescence Microscopy
Cells were cultured on coverslips and then fixed with 3% paraformaldehyde in phosphate-buffered saline. Samples were examined under a fluorescence laser scanning confocal microscope, FV1000 (Olympus).
Gel Filtration
Gel-filtration analysis was performed as previously described (4). Briefly, MEFs were homogenized in homogenization buffer (50 mm Tris-HCl (pH 7.5), 150 mm NaCl, and a protease inhibitor mixture (Roche Applied Science)) by repeatedly passing (∼15 times) through a 1-ml syringe with a 23-gauge needle. The homogenate was centrifuged at 10,000 × g for 10 min, and the supernatant was further centrifuged at 100,000 × g for 60 min. The resulting supernatants (cytosol fraction) were separated by size-exclusion chromatography on a Superose 6 column (Amersham Biosciences).
Sucrose Density Gradient Centrifugation
The cytosol fraction was prepared as described in the gel-filtration section. Two-hundred microliters of the cytosol fraction was loaded on the top of discontinuous stepwise sucrose gradients. The gradient was composed of 1 ml each of stepwise sucrose solutions (3, 6, 9, 12, 15, 18, 21, 24, 27, and 30%). The samples were sedimented for 16 h at 100,000 × g, and then 0.6-ml fractions were collected from the top of the gradient. Calibration of the gradient was performed using catalase, aldolase, and bovine serum albumin.
Bulk Protein Degradation Assay
The bulk protein degradation assay was performed as previously described (20).
Bacterial Infection
S. enterica serovar Typhimurium (SR-11 × 3181) was provided by the Research Institute of Microbial Disease, Osaka University (Osaka, Japan). Bacterial infections were performed as previously described (21). A colony forming assay was performed as follows. Cells were infected with S. typhimurium (multiplicity of infection = 100) for 10 min. After treating infected cells with gentamicin for 0, 2, or 6 h, the cells were lysed and serially diluted, and lysates were plated to determine the viable bacterial numbers.
Statistics
All values shown in the figures are represented with standard deviation.
RESULTS
Excess Atg16L1 Is Degraded
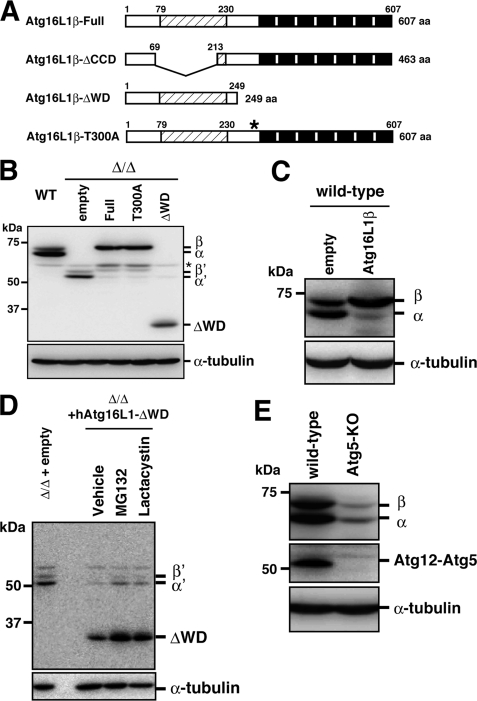
To examine the role of the WD repeat domain in Atg16L1, we constructed an Atg16L1 mutant that lacked the WD repeat domain and the T300A mutant (Fig. 1A). These mutants were stably expressed in Atg16L1-deficient (Δ/Δ) MEFs using a retrovirus expression system. We previously generated Atg16L1-deficient mice that are completely defective in autophagy (15). The MEFs derived from Atg16L1-Δ/Δ mice express deleted forms of the Atg16L1 protein that lack the entire coiled-coil domain, which is involved in Atg16L1 multimerization and is essential for Atg16L1 function (15) (Fig. 1A). Two truncated splicing variants, termed Atg16L1-α′ and β′, were detected in the MEF cell lysates by Western blotting with an anti-Atg16L1 antibody (15) (Fig. 1B). However, these bands were scarcely detectable in Atg16L1-Δ/ΔMEFs stably expressing exogenous wild-type Atg16L1, T300A, or the ΔWD mutant (Fig. 1B). Furthermore, although two spliced variants, Atg16L1-α and -β, were expressed in wild-type MEFs, the endogenous α form became the least detectable upon the exogenous overexpression of human Atg16L1-β (Fig. 1C). These results indicate that there is an upper limit of total Atg16L1 protein expression that is maintained within cells.
FIGURE 1.
Stability of Atg16L1 molecule. A, schematic diagram of Atg16L1 and the deletion constructs. The coiled-coil region is indicated by shading, and the WD repeats are shown as black boxes. The asterisk indicates the T300A mutation. B, wild-type or Atg16L1-Δ/ΔMEFs expressing the indicated constructs were cultured in growth medium and then harvested. Total cell lysates were examined by Western blotting using the indicated antibodies. Top panel, anti-Atg16L1; bottom panel, anti-α-tubulin. The asterisk indicates a nonspecific immunoreactive band. C, wild-type MEFs stably expressing the empty vector or full-length Atg16L1β were examined by Western blotting using the following antibodies: top panel, anti-Atg16L1; bottom panel, anti-α-tubulin. D, Atg16L1-Δ/ΔMEFs expressing the empty vector or Atg16L1-ΔWD were cultured in growth medium with DMSO (vehicle), 10 μg/ml MG132, or 20 μg/ml lactacystin for 6 h. Total cell lysate samples were examined by Western blotting using each antibody. Top panel, anti-Atg16L1; bottom panel, anti-α-tubulin. E, wild-type MEFs or Atg5 knockout MEFs were cultured in growth medium, and total cell lysates were examined by Western blotting using the following antibodies: top panel, anti-Atg16L1; middle panel, anti-Atg5; and bottom panel, anti-α-tubulin.
Treating with proteasome inhibitors increased the level of Atg16L1-α′, -β′, and ectopically expressed Atg16L1 proteins in Atg16L1-Δ/ΔMEFs that were stably expressing the exogenous Atg16L1β constructs (Fig. 1D and supplemental Fig. S1), suggesting that degradation by the ubiquitin-proteasome system helps control the Atg16L1 protein level. Because the Atg16L1 protein level was drastically reduced when Atg5 was knocked out (Fig. 1E and supplemental Fig. S2A), Atg16L1 molecules that were free from Atg12-Atg5 seemed to be unstable. We also observed that ectopic expression of GFP-Atg5 or treatment with the proteasome inhibitor increased the Atg16L1 protein level in Atg5 knockout MEFs (supplemental Fig. S2). Consistently, all of the Atg16L1 comigrates with the Atg12-Atg5 conjugate in a gel-filtration profile of wild-type cells (5). Thus, the total Atg16L1 protein level was tightly correlated with the amount of the Atg12-Atg5 conjugate. Because Atg16L1-ΔCCD had a lower affinity for the Atg12-Atg5 conjugate than Atg16L1, which contained the coiled-coil domain (supplemental Fig. S3), Atg16L1-ΔCCD was preferentially degraded in Atg16L1-reconstituted cells. Thus, we successfully obtained cells that expressed endogenous levels of the ΔWD or T300A mutant that were suitable for further analysis.
New Model of Atg16L1 Complex Organization
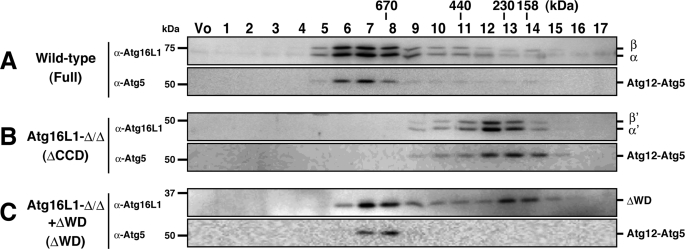
Next, we asked if the ΔWD mutant forms a multimeric complex like wild-type Atg16L1. The Atg12-Atg5 conjugate and Atg16L1 are supposed to form a multimer, probably an octamer, because both Atg16L1 (68 kDa) and the Atg12-Atg5 (50 kDa) conjugate eluted primarily in the ∼800-kDa fractions by size-exclusion chromatography (4) (Fig. 2A). The cytosol fraction of wild-type MEFs, Atg16L1-Δ/ΔMEFs (which express ΔCCD), and Atg16L1-Δ/ΔMEFs stably expressing Atg16L1-ΔWD were subjected to gel filtration. First, considering the molecular mass of the Atg12-Atg5 conjugate (∼50 kDa) and Atg16L1-ΔCCD (∼54 kDa), the complex in Atg16L1-Δ/Δ cells was expected to be ∼100 kDa. However, Atg16L1-ΔCCD eluted in fractions corresponding to ∼300 kDa (Fig. 2B). Second, although the molecular mass of Atg16L1-ΔWD (31 kDa) is much less than that of full-length Atg16L1 (68 kDa), the main fractions in which Atg16L1-ΔWD eluted were very similar to wild-type (Atg16L1-ΔWD molecules lacking Atg12-Atg5 conjugate were eluted in fractions 13 and 14 in Fig. 2C as a minor peak) (Fig. 2, A and C). These paradoxical results prompted us to closely examine the detailed organization of the Atg16L1 complex.
FIGURE 2.
Gel-filtration analysis of the Atg16L1 complex. Cytosolic fractions of wild-type MEFs (A), Atg16L1-Δ/ΔMEFs (B), or Atg16L1-Δ/ΔMEFs expressing Atg16L1-ΔWD (C) was separated by size-exclusion chromatography. Fractions were subjected to Western blotting using the indicated antibodies. The positions of the molecular mass standards are shown. Vo, void fraction.
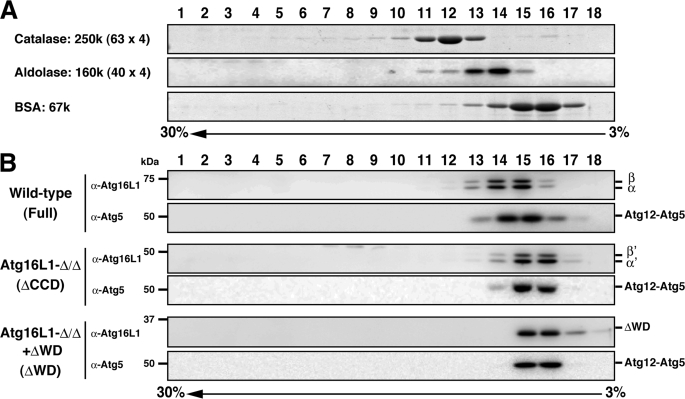
Gel filtration actually measures the Stokes radius (RS), a property that depends on the mass, shape, and hydration of a protein. Thus, using gel filtration as the only means to determine a molecular weight can be misleading. However, when a Stokes radius (RS) is measured by size-exclusion chromatography and a sedimentation coefficient (S) determined by density gradient centrifugation, the molecular weight of a protein can be calculated independent of its shape and hydration (22). To precisely determine the molecular weight of the Atg16L1 complex, we performed sucrose density gradient centrifugation. The apparent molecular weight of the Atg16L1 complex in the sucrose density gradient centrifugation was much less than that obtained from the gel-filtration analysis (Fig. 3 and Table 1). The difference in the apparent molecular weight between the two methods might be due to the high concentration of sucrose in the gradient. To exclude this possibility, Tris-buffered saline with 20% sucrose was used as the gel-filtration running buffer. As shown in supplemental Fig. S4, even in the presence of 20% sucrose, the Atg16L1 complex primarily eluted in fractions corresponding to ∼800 kDa.
FIGURE 3.
Sucrose density gradient analysis of the Atg16L1 complex. A, standard proteins were separated using discontinuous sucrose density gradient centrifugation as described under “Experimental Procedures.” Fractions were subjected to SDS-PAGE and stained with Coomassie Brilliant Blue. B, cytosolic fractions of wild-type MEFs, Atg16L1-Δ/ΔMEFs expressing the empty vector, or Atg16L1-ΔWD were separated using discontinuous sucrose density gradient centrifugation as in A. Fractions were subjected to Western blotting using the indicated antibodies.
TABLE 1.
Physicochemical properties of the Atg16L1 complex
Mtheor., theoretical molecular weight for a monomer; Mapp., apparent molecular weight; Mcalc., molecular weight calculated according to Siegel and Monty (equation: M = s × RS × 4.2).
| Construct | Mtheor., Atg16L1 (+Atg12–5) | Gel filtration |
Sucrose density gradient |
Mcalc. | ||
|---|---|---|---|---|---|---|
| Stokes radius | Mapp. | Sedimentation coefficient | Mapp. | |||
| kDa | Å | kDa | s | kDa | kDa | |
| Full | 68 (116) | 90 | 800 | 6.3 | 120 | 238 |
| ΔCCD | 54 (102) | 58 | 300 | 4.5 | 70 | 110 |
| ΔWD | 31 (79) | 86 | 700 | 4.5 | 70 | 162 |
The Stokes radius and sedimentation coefficient for the Atg16L1 complexes were determined from standard curves (supplemental Fig. S5 and Table 1). Then, the molecular weight of each complex was calculated using the Siegel and Monty equation (Table 1) (22). The calculated molecular mass of the Atg16L1-ΔCCD complex was nearly equal to the combined molecular weight of each single subunit. Furthermore, the calculated molecular mass of the Atg16L1 complex in both wild-type MEFs and Atg16L1-ΔWD-expressing MEFs was very close to double the molecular weight of the monomer. Therefore, we concluded that the Atg16L1 complex exists as a dimer and that the WD repeat domain is dispensable for this dimer formation.
Impact of Deleting the Atg16L1 WD Repeat Domain and the Crohn Disease-associated Mutation on Autophagy
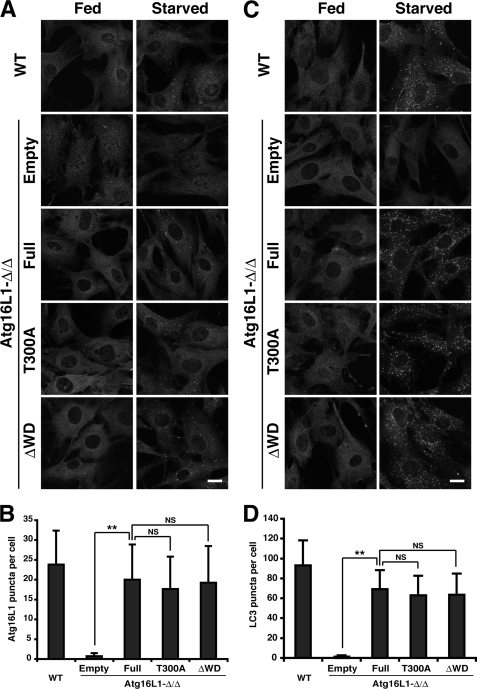
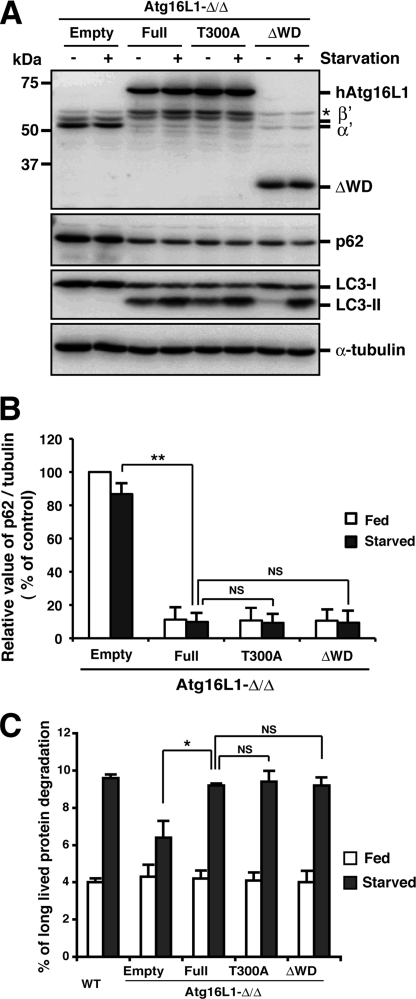
Based on these biochemical characterizations of the Atg16L1 complex, we confirmed that we successfully established an experimental system expressing an exogenous WD repeat domain mutant with a native organization. Next, we analyzed the role of the WD repeat domain. To explore the role of the WD repeat domain and the Crohn disease-associated point mutation (T300A), we immunostained for Atg16L1. Upon nutrient starvation, Atg16L1 puncta formed in Atg16L1-Δ/ΔMEFs stably expressing the T300A mutant or ΔWD to a similar extent as wild-type cells or full-length-reconstituted cells (Fig. 4, A and B), indicating that the WD repeat domain is dispensable for the localization of the Atg16L1 complex. The LC3 puncta also formed under starvation, and a significant difference was not observed with both WD repeat mutants (Fig. 4, C and D). The level of LC3-II formation was not significantly altered in starved condition, although the LC3-II level was decreased in the fed ΔWD mutant-reconstituted cells (Fig. 5A), indicating that the E3-like function of the Atg16L1 complex was hardly affected by both mutants.
FIGURE 4.
Impact of the Atg16L1 WD repeat deletion mutant and T300A mutant on autophagosome formation. Wild-type or Atg16L1-Δ/ΔMEFs expressing the indicated constructs were cultured in growth medium (Fed) or EBSS (Starved) for 1 h and subjected to immunocytochemistry using an anti-Atg16L1 (A) or anti-LC3 (C) antibody. An anti-rabbit antibody conjugated with Alexa488 was used as secondary antibody. The number of Atg16L1 (B) or LC3 (D) puncta in starved cells was counted in >70 cells. Each value represents the mean ± S.D. Statistical analysis was performed by Student's t test: **, p < 0.01; NS, not significant.
FIGURE 5.
Impact of the Atg16L1 WD repeat deletion mutant and T300A mutant on autophagic degradation. A and B, Atg16L1-Δ/ΔMEFs expressing the indicated constructs were cultured in growth medium (+) or EBSS (−) for 1 h and then collected. Total cell lysate samples were examined by Western blot using each antibody. From top to bottom, panels represent anti-Atg16L1, anti-p62, anti-LC3, and anti-α-tubulin. The asterisk indicates a nonspecific immunoreactive band (A). The graph indicates the ratio between the intensity of the p62 band versus α-tubulin band expressed as a percentage of that in fed empty reconstituted cells. The average ± S.D. is shown for two independent experiments. Statistical analysis was performed by using Student's t test: **, p < 0.01; NS, not significant (B). C, wild-type or Atg16L1-Δ/ΔMEFs expressing the indicated constructs were grown in growth medium (Fed) or EBSS (Starved) for 2 h. Long-lived protein degradation was scored by a standard protocol. Each value represents the mean ± S.D. Statistical analysis was performed by Student's t test: *, p < 0.05; NS, not significant.
Abnormal accumulation of p62 is a well known index for impaired basal autophagic activity (23). As shown in Fig. 5 (A and B), p62 accumulation in Atg16L1-Δ/Δ MEFs was suppressed by expression of the T300A mutant or ΔWD to almost the same extent as wild type. We also confirmed that the T300A mutation and WD repeat deletion mutant had little effect on starvation-induced autophagic flow in a bulk protein degradation assay (Fig. 5C). Collectively, we conclude that the WD repeat domain in Atg16L1 is dispensable for canonical autophagy.
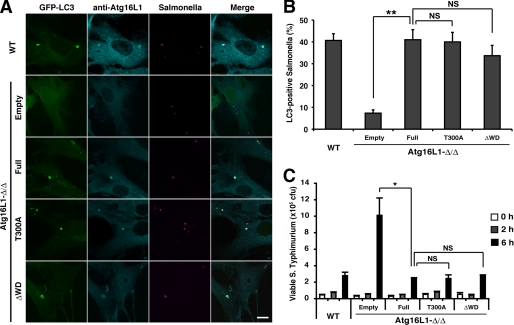
There is strong evidence that enteric microflora play a central role in the initiation and maintenance of Crohn disease (10). One function of autophagy in mammalian cells is to eliminate invasive microbes (3). Because the WD repeat domain is absent in yeast, it may be important for a specialized form of autophagy, such as an antibacterial function. To test this possibility, Atg16L1-reconstituted cells were challenged with S. typhimurium. We previously showed that Atg16L1-Δ/Δ macrophages fail to recruit LC3 around S. typhimurium. However, infection with S. typhimurium did not induce a marked difference in LC3 recruitment between full-length-, T300A mutant-, and ΔWD-reconstituted cells (Fig. 6, A and B). A similar result was obtained with group A Streptococcus challenge (supplemental Fig. S6). Although it has been reported that T300A mutant is less stable than wild type after bacterial challenge (13), the total T300A mutant protein level was not affected after S. typhimurium challenge in our experimental system (supplemental Fig. S7). In addition, there were no differences in the half-life among them (supplemental Fig. S8). The disagreement might be due to the difference in cell types that we used. To further examine the effect of the T300A mutant or WD repeat deletion on bacterial growth arrest, colony forming assays were performed. It has been previously reported that the loss of autophagy enhances S. typhimurium replication within cells (21). Consistent with this finding, Atg16L1-Δ/ΔMEFs harbored significantly more viable bacteria than wild-type cells. However, we observed no significant differences between full-length-, T300A mutant-, and ΔWD-reconstituted cells (Fig. 6C). Collectively, the results clearly indicate the autophagic process against invading bacteria proceeds normally with the T300A and ΔWD mutants.
FIGURE 6.
Impact of the Atg16L1 WD repeat deletion mutant and T300A mutant on bacterial growth arrest. A and B, wild-type or Atg16L1-Δ/ΔMEFs expressing GFP-LC3 and the indicated constructs were infected with mCherry expressing S. typhimurium (multiplicity of infection = 10) for 1 h, and then analyzed by immunocytochemistry for Atg16L1 (A). The percentage of LC3-positive bacteria was enumerated by fluorescence microscopy. At least 50 bacteria were counted. The average ± S.D. is shown for three independent experiments. Statistical analysis was performed by using Student's t test: **, p < 0.01; NS, not significant. C, wild-type or Atg16L1-Δ/ΔMEFs expressing the indicated constructs were infected with S. typhimurium (multiplicity of infection = 100) for 10 min. After treating with gentamicin for 0, 2, or 6 h, the cells were lysed and serially diluted, and the lysates were plated to determine the number of viable bacteria. Each value represents the mean ± S.D. Statistical analysis was performed by using Student's t test: *, p < 0.05; NS, not significant.
DISCUSSION
In this study, we characterized the biochemical properties of the Atg16L1 complex to establish a reliable experimental system that can be used to explore the function of the WD repeat domain in Atg16L1. Using Atg16L1-deficient cells, we revealed that Atg16L1 and the Atg12-Atg5 conjugate form a dimeric complex and that the total Atg16L1 protein level is strictly maintained, possibly by the ubiquitin-proteasome system.
Unassembled Atg16L1 protein is eliminated, suggesting that Atg16L1 molecule functions as a complex with Atg12-Atg5 conjugate and may not have a role outside the complex. Based on the perspective that cells want to maximize their amino acid resources, it is reasonable to assume that cells would recycle surplus non-functional subunits. Furthermore, this degradation mechanism is important for efficient autophagy, because abrupt overexpression of Atg16L1 by an adenovirus expression system severely inhibits autophagy, possibly by titrating out some interactor (5). This kind of fine-tuning of the molecular abundance may be one of the general roles of the ubiquitin-proteasome system, and molecular chaperones such as HSP90 may participate in these processes (24).
The Siegel and Monty equation provided a highly rational model for the organization of the Atg16L1 complex. The discrepancy between the Stokes radius in a gel-filtration analysis and the calculated value from the Siegel and Monty equation is potentially caused by the Atg16L1 complex having an elongated rather than a globular shape. The three-dimensional structure of the N-terminal region of yeast Atg16 bound to the Atg12-Atg5 conjugate has been reported (25). Atg5 contains two ubiquitin fold domains, and Atg12, which also contains a ubiquitin fold, is conjugated in between these domains (26). Because deleting the WD repeat domain does not lead to a major decrease in the Stokes radius compared with full-length Atg16L1, we speculate that Atg16L1 forms an antiparallel dimer. In this state, the Atg16L1 complex takes on a cylindrical shape, possessing three ubiquitin folds at the both ends.
We have clearly shown that the WD repeat domain is dispensable for canonical autophagy and antibacterial autophagy in fibroblasts. This finding is surprising, because the WD repeat domain is highly conserved in multicellular eukaryotes, including Caenorhabditis elegans and plants. This high conservation suggests that the WD repeat domain performs a general role in addition to the phenomena observed in this study. Alternatively, the WD repeat domain might have an important role in some specific cell types but not in fibroblasts. Also, we cannot rule out the possibility that the T300A mutation has an impact only on the α-form, because we used β-form constructs in this study.
Starvation-induced autophagosome formation, p62 degradation, and long-lived protein degradation were hardly affected by T300A or ΔWD mutants (Figs. 4 and 5), however, the LC3-II level was decreased in fed ΔWD mutant-reconstituted cells (Fig. 5A). This might be due to a slight reduction in the E3-like function by deletion of WD repeat domain or the WD repeat domain having a role in the fed condition.
It is not clear how the T300A mutation affects Crohn disease besides canonical autophagy. Because the frequency of the Crohn disease-associated allele is relatively high, even in healthy controls (0.60 in affected individuals and 0.53 in controls) (7), the T300A mutation may not have a significant effect on the role of the WD repeat domain. Another possibility is that the Atg16L1 complex is involved in a specialized pathway other than autophagy. Cadwell and colleagues reported that Atg16L1-hypomorphic mice did not exhibit significant deficiencies in managing Listeria monocytogenes infections, despite a remarkable defect in paneth cells (27). Paneth cells exocytose granules containing antimicrobial products, such as α-defensins, lysozyme, and secretory phospholipase A2. The Atg16L1 complex might be involved in the granule exocytosis pathway, and the Crohn disease-associated mutation might impact this pathway.
In this study we generated a rigid platform that is essential to further understand Crohn disease and the function of the Atg16L1 complex. It is highly possible that the T300A mutation causes subtle effects, and we hope this study helps shed further light on these differences.
Supplementary Material
Acknowledgments
We thank Dr. Roger Y. Tsien (University of California, San Diego, CA) for kindly providing the mCherry cDNA; Dr. Shoji Yamaoka (Tokyo Medical and Dental University, Japan) for the pMRX-IRES-puro and pMRX-IRES-bsr; Dr. Toshio Kitamura (University of Tokyo, Japan) for the Plat-E cells; Dr. Noboru Mizushima (Tokyo Medical and Dental University, Japan) for the anti-Atg16L1 antibody; Dr. Maho Hamasaki for critical reading of the manuscript; and all of the members of the Yoshimori laboratory for helpful discussions.
This work was supported in part by the Ministry of Education, Culture, Sports, Science, and Technology (to T. Y. and T. N.), the Japan Society for the Promotion of Science (to N. F.), and the Takeda Science Foundation (to T. Y.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S8.
- E3
- ubiquitin-protein isopeptide ligase
- EBSS
- Earle's balanced salt solution
- MEF
- mouse embryonic fibroblast
- S. Typhimurium
- Salmonella enterica serovar Typhimurium
- RS
- Stokes radius
- S
- sedimentation coefficient.
REFERENCES
- 1.Yoshimori T., Noda T. (2008) Curr. Opin. Cell Biol. 20, 401–407 [DOI] [PubMed] [Google Scholar]
- 2.Mizushima N., Levine B., Cuervo A. M., Klionsky D. J. (2008) Nature 451, 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virgin H. W., Levine B. (2009) Nat. Immunol. 10, 461–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizushima N., Kuma A., Kobayashi Y., Yamamoto A., Matsubae M., Takao T., Natsume T., Ohsumi Y., Yoshimori T. (2003) J. Cell Sci. 116, 1679–1688 [DOI] [PubMed] [Google Scholar]
- 5.Fujita N., Itoh T., Omori H., Fukuda M., Noda T., Yoshimori T. (2008) Mol. Biol. Cell 19, 2092–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanada T., Noda N. N., Satomi Y., Ichimura Y., Fujioka Y., Takao T., Inagaki F., Ohsumi Y. (2007) J. Biol. Chem. 282, 37298–37302 [DOI] [PubMed] [Google Scholar]
- 7.Hampe J., Franke A., Rosenstiel P., Till A., Teuber M., Huse K., Albrecht M., Mayr G., De La Vega F. M., Briggs J., Günther S., Prescott N. J., Onnie C. M., Häsler R., Sipos B., Fölsch U. R., Lengauer T., Platzer M., Mathew C. G., Krawczak M., Schreiber S. (2007) Nat. Genet. 39, 207–211 [DOI] [PubMed] [Google Scholar]
- 8.Rioux J. D., Xavier R. J., Taylor K. D., Silverberg M. S., Goyette P., Huett A., Green T., Kuballa P., Barmada M. M., Datta L. W., Shugart Y. Y., Griffiths A. M., Targan S. R., Ippoliti A. F., Bernard E. J., Mei L., Nicolae D. L., Regueiro M., Schumm L. P., Steinhart A. H., Rotter J. I., Duerr R. H., Cho J. H., Daly M. J., Brant S. R. (2007) Nat. Genet. 39, 596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wellcome Trust Case Control Consortium ( 2007) Nature 447, 661– 67817554300 [Google Scholar]
- 10.Xavier R. J., Podolsky D. K. (2007) Nature 448, 427–434 [DOI] [PubMed] [Google Scholar]
- 11.Kanneganti T. D., Lamkanfi M., Núñez G. (2007) Immunity 27, 549–559 [DOI] [PubMed] [Google Scholar]
- 12.Singh S. B., Davis A. S., Taylor G. A., Deretic V. (2006) Science 313, 1438–1441 [DOI] [PubMed] [Google Scholar]
- 13.Kuballa P., Huett A., Rioux J. D., Daly M. J., Xavier R. J. (2008) PLoS ONE 3, e3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosokawa N., Hara Y., Mizushima N. ( 2007) FEBS Lett. 581, 2623– 2629 [DOI] [PubMed] [Google Scholar]
- 15.Saitoh T., Fujita N., Jang M. H., Uematsu S., Yang B. G., Satoh T., Omori H., Noda T., Yamamoto N., Komatsu M., Tanaka K., Kawai T., Tsujimura T., Takeuchi O., Yoshimori T., Akira S. (2008) Nature 456, 264–268 [DOI] [PubMed] [Google Scholar]
- 16.Mizushima N., Yamamoto A., Hatano M., Kobayashi Y., Kabeya Y., Suzuki K., Tokuhisa T., Ohsumi Y., Yoshimori T. (2001) J. Cell Biol. 152, 657–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saitoh T., Nakayama M., Nakano H., Yagita H., Yamamoto N., Yamaoka S. (2003) J. Biol. Chem. 278, 36005–36012 [DOI] [PubMed] [Google Scholar]
- 18.Morita S., Kojima T., Kitamura T. (2000) Gene Ther. 7, 1063–1066 [DOI] [PubMed] [Google Scholar]
- 19.Sou Y. S., Tanida I., Komatsu M., Ueno T., Kominami E. (2006) J. Biol. Chem. 281, 3017–3024 [DOI] [PubMed] [Google Scholar]
- 20.Fujita N., Hayashi-Nishino M., Fukumoto H., Omori H., Yamamoto A., Noda T., Yoshimori T. (2008) Mol. Biol. Cell 19, 4651–4659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birmingham C. L., Smith A. C., Bakowski M. A., Yoshimori T., Brumell J. H. (2006) J. Biol. Chem. 281, 11374–11383 [DOI] [PubMed] [Google Scholar]
- 22.Siegel L. M., Monty K. J. (1966) Biochim. Biophys. Acta 112, 346–362 [DOI] [PubMed] [Google Scholar]
- 23.Kimura S., Fujita N., Noda T., Yoshimori T. (2009) Methods Enzymol. 452, 1–12 [DOI] [PubMed] [Google Scholar]
- 24.McClellan A. J., Scott M. D., Frydman J. (2005) Cell 121, 739–748 [DOI] [PubMed] [Google Scholar]
- 25.Matsushita M., Suzuki N. N., Obara K., Fujioka Y., Ohsumi Y., Inagaki F. (2007) J. Biol. Chem. 282, 6763–6772 [DOI] [PubMed] [Google Scholar]
- 26.Mizushima N., Noda T., Yoshimori T., Tanaka Y., Ishii T., George M. D., Klionsky D. J., Ohsumi M., Ohsumi Y. (1998) Nature 395, 395–398 [DOI] [PubMed] [Google Scholar]
- 27.Cadwell K., Liu J. Y., Brown S. L., Miyoshi H., Loh J., Lennerz J. K., Kishi C., Kc W., Carrero J. A., Hunt S., Stone C. D., Brunt E. M., Xavier R. J., Sleckman B. P., Li E., Mizushima N., Stappenbeck T. S., Virgin H. W., 4th (2008) Nature 456, 259–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.