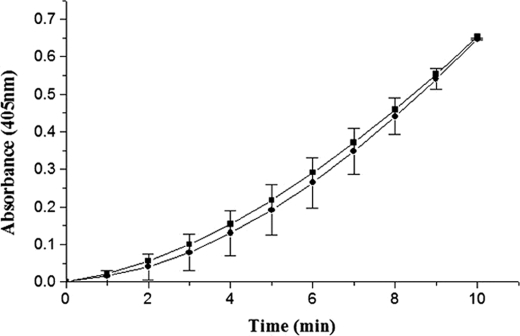
FIGURE 5.
Time course of the amidolytic activity generation in R561A μPG by wtSK and K180A mutant. Equimolar SK·R561A μPG mutant and K180A·R561A μPG mutant complexes were made, and catalytic amounts were added to the assay buffer containing 1 mm chromogenic substrate Chromozym®PL, 50 mm Tris, pH 7.4, 100 mm NaCl, and amidolytic activity generated was represented as wild-type SK (solid squares) or K180A mutant (solid circles). Assays were performed at 37 °C with 10 nm SK and μPG complex as described under “Experimental Procedures.”