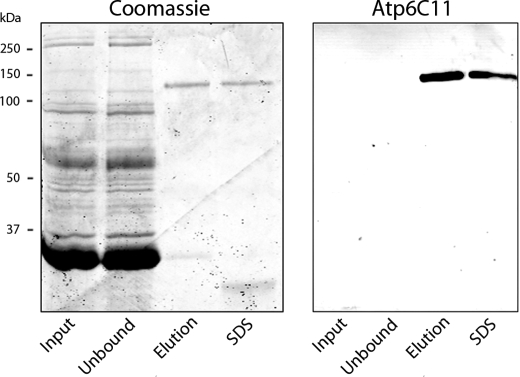
FIGURE 5.
Purification of Atp8a2 from bovine ROS membranes by immunoaffinity chromatography. ROS membranes solubilized in CHAPS detergent (precolumn) were applied to an Atp6C11-Sepharose column. The flow-through (unbound) and bound Atp6C11 eluted first with the competing 6C11 peptide (peptide) and subsequently with SDS were resolved by SDS gel electrophoresis. Gels were stained with Coomassie Blue, and Western blots were labeled with the Atp6C11 antibody to Atp8a2. The following amount of protein was applied to the indicated lanes: precolumn (30 μg), unbound (30 μg), peptide elution (100 ng), and SDS elution (100 ng).