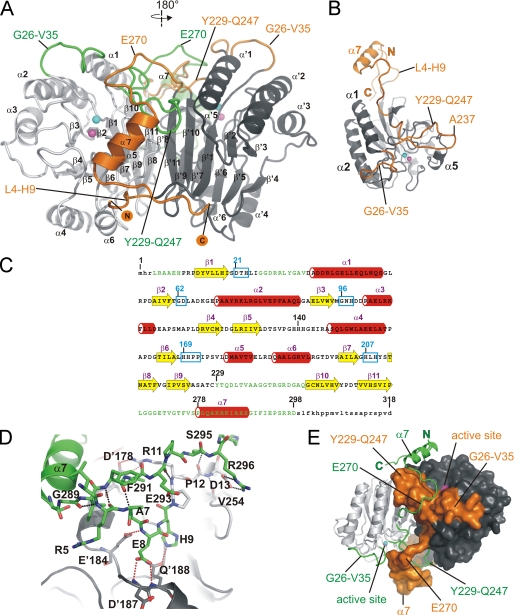
FIGURE 1.
Crystal structure of the Rv08051–318 homodimer. A, ribbon representation of the Rv08051–318 dimer. The MPE catalytic core is colored light gray (left-side protomer) or dark gray (right-side protomer). New structural elements are colored orange (attached to the dark gray catalytic core) or green (attached to the light gray catalytic core). N and C termini of the dark gray/orange protomer are marked. α-Helices and β-sheets are indicated, dark gray/orange protomer with a prime. Active site metals ions are shown as spheres: Fe3+, cyan; Mn2+, magenta. B, Rv08051–318 monomer: catalytic core, dark gray; new structural elements, orange. Active site metals are shown as in A. C, secondary structure elements of Rv08051–318 monomer superimposed onto the amino acid sequence (β-strands, yellow arrows; α-helices, red cylinders). Newly defined parts of the Rv08051–318 structure are in green. Highly conserved MPE family regions are highlighted by cyan boxes and several residues indicated in the text are numbered. Small letters represent parts of the structure that are not defined by electron density. D, polar interactions: between N-terminal and C-terminal residues of the same protomer (black dashed lines); between N-terminal peptide of one protomer and residues of the other protomer (red dashed line). Residues of the protomer in dark gray/orange combination are marked with a prime (i.e. D′187) and the light gray/green protomer as normal (i.e. D13). E, swapped structural elements between the protomers in the Rv08051–318 dimer. Color code is the same as in A. Protomer colored dark gray/orange is shown as a surface and the light gray/green protomer as ribbon.