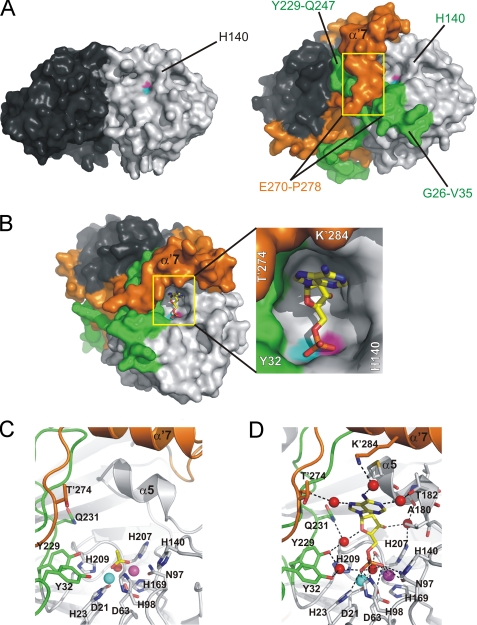
FIGURE 3.
The active site of Rv0805. A, comparison of Rv08051–278 (left) and Rv08051–318 (right) dimer shows a significant closure of the active site in Rv08051–318. In this view, only one of the two equal active sites is seen (light gray/green protomer). Yellow rectangles cover the regions of residues Glu′270-Pro′278 that run into α′7 helix from the bottom up. B, Rv08051–318 active site with bound 5′-AMP (left panel). 5′-AMP is shown as sticks (carbon, yellow; oxygen, red; nitrogen, blue; phosphorus, orange). Middle panel, zoom of the active site. C, zoom of the active site showing acetate in sticks bound to Rv08051–318 (carbon, yellow; oxygen, red; spheres are Fe3+, cyan; Mn2+, magenta; planar active site water, red). D, 5′-AMP-Rv08051–318 complex. For the 5′-AMP complex, polar interactions with the active site residues, metals, and water molecules are shown. Color code is the same as described in the legend to Fig. 1, residues of the protomer in dark gray/orange combination are marked with a prime.