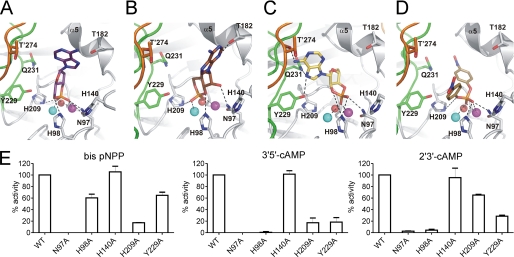
FIGURE 4.
Docking of substrates into the active site of Rv08051–318 dimer. A, 3′,5′-cAMP in orientation that releases 3′-AMP upon hydrolysis. B, 3′,5′-cAMP in orientation that releases 5′-AMP upon hydrolysis. C, 2′,3′-cAMP in orientation that releases 3′-AMP upon hydrolysis. D, bis-pNPP in an orientation that releases pNP upon hydrolysis. In all panels, potential polar interactions are depicted as dashed black lines. Color code for the protein is the same as in Fig. 1. Substrates are shown as sticks. Oxygen, red; nitrogen, blue; phosphorus, orange; carbon, violet, 3′,5′-cAMP in A; brown, 3′,5′-cAMP in B; yellow, 2′,3′-cAMP in C; dark yellow, bis-pNPP in D. Fe3+ (cyan), Mn2+ (magenta), and planar active site water (red) are shown as spheres. Residues of the protomer in dark gray/orange combination are marked with a prime. E, wild type or mutant Rv08051–318 proteins were assayed with the substrates indicated. Values represent the mean ± S.E. of assays repeated three times in duplicate, with two different batches of proteins, and are normalized to the activity seen with the wild type protein.