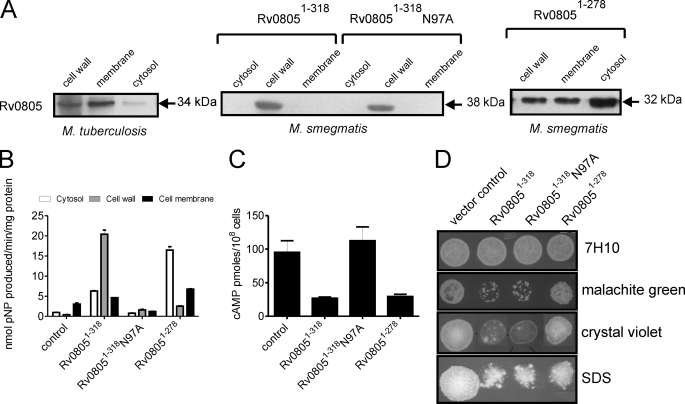
FIGURE 5.
Subcellular localization and function of Rv08051–318 and Rv08051–278 in mycobacteria. A, subcellular fractions (50 μg of protein) of M. tuberculosis were subjected to Western blot analysis using a monoclonal antibody to Rv0805. His-tagged wild type or mutant Rv08051–318 or Rv08051–278 were expressed in M. smegmatis and subcellular fractions prepared. Protein (50 μg) was subjected to Western blot analysis using a monoclonal antibody to Rv0805. The molecular sizes indicated include the addition of additional residues coming from the His6 tag and vector. B, protein (50 μg) was taken for assay using bis-pNPP as a substrate. Note that expression of Rv08051–278 was higher in cells than that of Rv08051–318 (panel A), but the activity with bis-pNPP is lower for Rv08051–278 than Rv08051–318 (see Table 2). Subcellular fractions prepared from a strain of M. smegmatis transformed with the control vector (control) was used to detect background activity. C, intracellular cAMP was measured in a control strain of M. smegmatis, and in strains expressing wild type or mutant Rv08051–318 proteins or Rv08051–278. Cells were harvested at late-log phase for cAMP measurements. D, cells (107) of the indicated strains were spotted on 7H10 agar plates containing 5 μg/ml of malachite green, 10 μg/ml of crystal violet, or 0.01% SDS and incubated for 2–3 days, after which the plates were photographed.