Abstract
The environment in which a breeding female lives prior to conception and during the early stages of her pregnancy has striking effects on oocytes developing in the ovarian follicle and on early embryos in the reproductive tract. Of the various environmental factors known to affect oocyte and embryo development, altered nutrition during this critical period has been particularly well studied. Alterations in the quantity of food consumed or the composition of the diet imposed solely during the pre-mating period affect oocyte maturity, blastocyst yield, prenatal survival and the number of offspring born alive. Importantly, nutrition at this time also affects the quality of embryos and resultant offspring, with increasing evidence from a variety of species showing that peri-conception nutrition can alter behaviour, cardiovascular function and reproductive function throughout post-natal life. In livestock species, it is important to devise nutritional strategies that improve reproductive efficiency and the quality of offspring but that do not add to the environmental footprint of the production system and which recognize likely changes in feedstuff availability arising from predicted changes in climate.
Keywords: nutrition, oocyte, embryo, foetus, environment
1. Introduction
This paper will consider the effects of environmental change on the development of oocytes and embryos in the female reproductive tract and the long-term consequences of such environmentally induced changes in terms of both the well-being of the resultant offspring and the impact of changes in reproductive fitness on the environment. In addition, the impact of predicted changes in feedstuff availability as a consequence of environmental change on reproductive success will be discussed.
Superficially, it may appear that the oocyte developing in the ovarian follicle and the embryo within the oviduct or uterus are well removed from changes in the external environment and therefore enjoy a protected existence. In fact, many studies have shown that the period when the oocyte is maturing in the ovarian follicle and the first few weeks of embryo development are particularly sensitive to changes in the maternal environment reflecting changes in the external world. Changes in the maternal environment include alterations in nutrient intake, diet composition, temperature, health status, maternal stress and environmental pollution. For example, recent data show that mice subjected prior to conception to fine particulate matter emitted by traffic had higher levels of prenatal death and carried lighter foetuses compared with mice subjected to filtered air during the same period (Veras et al. 2009).
This review will focus primarily on nutrition as the environmental factor affecting oocytes and embryos, although reference will be made to the impact of other environmental factors. It will address the impact of altered nutrient supply during the peri-conceptual period firstly on immediate or short-term effects on the developing oocytes and early embryos and secondly on longer term ‘programming’ effects of altered nutrient supply at this stage on specific foetal and neonatal organ systems and functions. Both the amount of food consumed and the composition of the diet are important. Most examples will be drawn from livestock species, where the impact of production systems on the environmental footprint is a topic of much concern and speculation. However, from a biological perspective, the overarching principles are believed to be similar among many mammals.
While the underlying mechanisms whereby nutritional status affects oocyte and embryo development are not fully elucidated, they are believed to involve nutritionally induced alterations in hormone and intermediary metabolites in the female body, which alter the biochemical properties of the ovarian follicle and genital tract where the oocyte and embryo, respectively, develop. Changes to the immediate environment surrounding oocytes and embryos can alter the pattern of genes expressed by these structures, with consequences for both immediate and longer-term development. There is also considerable interest in how environmental factors induce epigenetic modifications, that is, heritable changes in gene function that occur without an alteration in DNA sequence, to oocytes and embryos which in turn contribute to altered developmental potential (Burdge et al. 2007).
2. Changes to the maternal environment affect the earliest stages of development
One of the predicted consequences of climate change is an increase in average temperatures in many parts of the world. Studies investigating factors associated with seasonal infertility in tropical and sub-tropical regions reveal that embryo development is sensitive to transient increases in body temperature arising as a consequence of elevated temperature. Using an integrated temperature humidity index, which is an integrated measure of heat comfort, Benyei et al. (2003) showed that heat stress during El Nino periods was associated with a reduction in the average number of embryos recovered from donor cows, the proportion of live embryos and the quality of live embryos. Heat stress affects many components of the reproductive system including gonadotrophin profiles, follicular growth, granulosa cell function, steroidogenesis and oocyte and embryo development (reviewed by Roth 2008). Many studies on the effect of heat stress on fertility have been conducted using dairy cattle. Increasing milk yields make it more difficult for cows to regulate body temperature during warm weather and hence exacerbate effects of heat stress on fertility (Al-Katanani et al. 1999). Only 21 per cent of embryos were normal when recovered from dairy heifers exposed to 30°C for up to 16 h each day and to 42°C for the remaining 8 h of the first 7 days after artificial insemination, compared to 52 per cent from heifers kept at control (20°C) temperatures (Putney et al. 1988). Heat-stressed heifers had a higher incidence of abnormal and retarded embryos with degenerative blastomeres, even though rectal temperature was only elevated (to 41.1°C) during the 8 h of exposure to 42°C. Increasing the temperature to which beef cattle were exposed to 37°C for 12 h and 33°C for 12 h between days 7 and 16 of pregnancy did not alter pregnancy rates, but resulted in reduced total conceptus mass compared with cows at 22°C (Biggers et al. 1987), suggesting that some embryo mortality occurred in heat-stressed cows. Interestingly, although increased humidity during this period altered metabolic and respiratory traits, it had no effect on conceptus mass or pregnancy rates. Conceptuses recovered from cows subjected to such treatments secreted more of the luteotrophic factor interferon (IFN)τ in vitro than conceptuses from cows housed at 21°C (Geisert et al. 1998).
Following these in vivo observations, cleavage-stage embryos and blastocysts recovered from a variety of mammalian species have been cultured at elevated temperatures to identify the nature of heat-stress-induced perturbations. Exposure to in vitro heat stress induces a range of effects including increased apoptosis in bovine (Paula-Lopes & Hansen 2002) and rabbit (Makarevich et al. 2007) embryos, increased expression of heat shock proteins by porcine (Bernardini et al. 2004) and mouse (Kim et al. 2002) embryos, disruption of microtubule and microfilaments (Rivera et al. 2004) and alterations in the methylation status of imprinted genes including H19 and insulin-like growth factor (Igf-2r) in mouse embryos (Zhu et al. 2008).
More recently, observations of impaired fertility of dairy cattle in the autumn months, following a hot summer, led to greater awareness of the sensitivity of the ovarian follicle and its developing oocyte to heat stress (reviewed by Roth 2008). Oocytes collected from cows during the summer have a reduced ability to develop to the blastocyst stage after in vitro fertilization (Al-Katanani et al. 2002). However, heat stress affects not only antral follicles emerging in the follicular wave, but also the ovarian pool of small antral follicles, resulting in carry-over effects on follicular function and oocyte developmental competence. Recent studies of in vitro matured mouse oocytes indicate that oocyte cytoplasmic maturation (migration of cortical granules and mitochondria) is less tolerant to heat stress than nuclear maturation (development to metaphase II) (Wang et al. 2009).
3. Effects of altered nutrient supply
Relatively short-term changes in the quantity or composition of the diet at key stages in the reproductive process provide new and acceptable means to improve reproductive efficiency while minimizing environmental cost. The term ‘focus feeding’ is based on using short periods of nutritional supplements that are precisely timed and specifically designed for stages of the reproductive process (Martin & Kadokawa 2006). Judicial ‘focus feeding’ provides opportunities to enhance reproductive capability in both the generation of animals consuming the diet (through improvement in gamete quality and embryo competence) and their offspring (through alterations to developing foetal gonads).
4. Short-term effects
It is now evident that nutritional effects on oocyte quality can originate when ovarian follicles emerge from the primordial pool and become committed to growth (approx. six months before they ovulate in ewes and three to four months in cows). Undernutrition at this time reduces the number of follicles that emerge and therefore the number available to ovulate (from data reviewed by Robinson et al. 2002).
(a). Ovulation rate
The vast majority of studies investigating the relationship between feed intake and ovulation rate have been conducted in livestock species where the positive relationship between increased feed intake and ovulation rate is well documented. Recent data suggest that in sheep, the critical window for the stimulatory effect of improved nutrition could be as short as 5 days, if it coincides with the emergence of the ovulatory follicular wave (e.g. between 8 and 4 days before ovulation). The effects of diet type and the endproducts of digestion on the ovulatory response in sheep have been recently reviewed (Robinson et al. 2006). Within species, the ovulatory response to a common nutritional change can vary between breeds and genotypes. For example, studies using Booroola Merino ewes showed higher ovulation rates in carriers of the FecB fecundity gene following an increased starch diet for three weeks prior to ovulation, but this increase did not occur in non-carriers of the gene (Landau et al. 1995). Such genotype-dependent differences in response to a specific dietary component bring a new dimension to the focus feeding approach described above and raise important issues when considering optimal uses of nutritional resources to improve lifetime reproductive performance.
(b). Oocyte maturity
Nutrition affects not only the number of oocytes that ovulate but also their quality. While the only definitive measure of oocyte quality is its ability to form a blastocyst, and indeed viable young, numerous proxy measures of oocyte quality are used, including the attainment of metaphase II following in vitro maturation and the expression of key genes. In pigs, consumption of increased amounts of feed (Zak et al. 1997; Ferguson et al. 2003) or increased dietary fibre (Ferguson et al. 2007) for 19 days immediately preceding oocyte recovery and in vitro assessment increased the number of oocytes in metaphase II. Recent data (Pisani et al. 2008) indicate that feeding ewes 0.5 maintenance requirements for two weeks altered the relative abundance of transcripts involved in oocyte metabolic activity. Specifically, such short-term nutrient restriction reduced expression of glucose transporter 3 (SLC2A3), sodium/glucose co-transporter 1 (SLC5A1) and Na+/K+ ATPase mRNA in oocytes, while expression of PTGS2, HAS2 and the leptin receptor long form in granulosa cells was increased. Reduced expression of SLC2A3 is potentially relevant in the light of data showing that it is essential for post-implantation embryonic development (Schmidt et al. 2009).
Much of our understanding of the relationships between nutrition, oocyte quality and blastocyst yield comes from studies on the causes of reduced fertility in the high-yielding dairy cow. In such animals, the excessive negative energy balance of early lactation coincides with a critical window in oocyte development. At least part of the explanation for decreased fertility in many dairy cows may be the conflicting effects of changes in metabolite or hormone concentrations on the developing follicle-enclosed oocyte that may not be compatible with optimal ovarian and subsequent embryonic development. Studies of non-lactating heifers, which enable investigations of nutritional effects on oocyte quality in the absence of negative energy balance, showed that the effect of feeding level on oocyte quality, as assessed by blastocyst yield on day 8, depended upon the body condition of the animal: high levels (2 × maintenance rations) of feeding were beneficial to animals of low body condition, but detrimental to oocytes from animals of moderately high body condition (Adamiak et al. 2005). These authors also reported that hyperinsulinaemia was associated with impaired oocyte quality in cattle. The effects of changing dietary carbohydrate (fibre versus starch) or supplementing the diet with fatty acids on oocyte quality were more modest (Adamiak et al. 2006). More recent data (Rooke et al. 2009) show that a high starch diet, which was associated with a higher plasma insulin : glucagon ratio, had adverse effects on oocyte quality but these were avoided when leucine intake was increased. Therefore, in dairy heifers; dietary amino acids and carbohydrates during antral follicle development appear to mediate effects on oocyte quality by different mechanisms.
(c). Blastocyst development and function
Studies investigating the effects of altered nutrition on blastocyst development have either altered the amount or the composition of the maternal diet before and/or after fertilization or they have modified the composition of media used for in vitro oocyte and/or embryo culture. Such studies have provided new information on the nutrient needs of developing embryos and have shed light on possible mechanisms whereby altered nutrient supply affects immediate and later development.
Data from several species show that pre-mating diets that improve oocyte maturity are also associated with improvements in embryo survival, even when animals were fed control diets after mating. Examples include increased pre-mating feed intake in pigs (Zak et al. 1997; Ferguson et al. 2003) and feeding a high-fibre diet to gilts during the oestrous cycle preceding mating (Ferguson et al. 2007). In sheep, ewes that were fed 60 per cent of control rations for eight weeks prior to oocyte collection had poorer quality oocytes and lower rates of blastocyst formation (Borowczyk et al. 2006). Collectively, these results suggest that pre-mating diets that affect embryo survival do so, at least in part, by altering the quality of the oocyte. Further support for this notion comes from the observation that blastocyst yields following in vitro fertilization and culture were higher when the oocytes were from gilts fed the high fibre diet, compared with oocytes from control-fed gilts (Ashworth et al. 2008).
Much of our understanding of the relationship between nutrient supply and embryo gene expression comes from in vitro studies in which embryos have been cultured in media of differing nutrient composition. Expression of a wide range of genes is affected by culture conditions, including genes involved in the adaptation to stress, metabolism, trophoblast function, DNA methylation, growth factors, cytokine signalling, apoptosis and compaction (reviewed by Ashworth et al. 2005). Of more relevance to the current topic are observations that the composition of the diet consumed by females around the time of conception can alter the expression of key developmental genes in the developing embryo. For example, feeding a low protein diet (9% casein versus 18% casein) during the pre-implantation period in rats reduced expression of the growth-regulating imprinted gene H19mRNA in male, but not female, days 2–4 blastocysts (Kwong et al. 2006). This response was also evident in the livers from late gestation foetuses carried by mothers fed the low protein diet for the first 4.25 days of gestation, demonstrating that short-term changes in nutrient supply around the time of conception can induce both immediate and longer term changes on conceptus gene expression that may be associated with altered post-natal growth.
It is of interest that pre-mating diets that increase embryo survival also increase blastocyst cell number (increased feed intake: Ashworth et al. 1999; high fibre diet: Ashworth et al. 2008), suggesting that this may be one mechanism by which such pre-mating diets exert their beneficial effects. Similar positive associations have been observed following micronutrient deficiency and in rodent species. For example, embryos recovered from mice fed a zinc-deficient diet for a 3- or 6-day period encompassing ooctye maturation and fertilization had fewer cells and delayed blastocyst development in vitro (Peters et al. 1991). Zinc supplementation of the media used to culture pre-implantation mouse embryos recovered from dams fed a zinc-deficient diet for 3 days from the day before mating did not improve blastocyst development. These data demonstrate that, at least with respect to the micronutrient zinc, the effects of peri-conceptual deficiency cannot be overcome by subsequent supplementation.
Maternal nutritional status also affects embryo metabolism. In many cases, increases in embryo metabolic activity, particularly glucose metabolism, are considered a hallmark of cellular stress (Sviderskaya et al. 1996). For example, in a study designed to examine the effects of increased plasma ammonia and its metabolites on embryo development, day 3 embryos recovered from ewes fed supplementary urea for 12 weeks were developmentally retarded compared with control embryos, they used more glucose when cultured in vitro and some exhibited up to a 2.8-fold increase in metabolism (McEvoy et al. 1997).
Oocytes recovered from mice fed a diet relatively high in long-chain n-3 polyunsaturated fatty acids for four weeks immediately preceding collection exhibited altered mitochondrial distribution and calcium levels and increased production of reactive oxygen species. The embryos resulting from fertilization of these oocytes had poorer morphology and decreased developmental ability to the blastocyst stage (Wakefield et al. 2008).
Some of the most intriguing data to emerge from studies of nutrient effects on embryo development in recent years are those showing that the gender of an embryo affects its susceptibility to altered pre-mating nutrition. Studies in which primiparous sows were fed a restricted diet (approx. 50% maintenance) during the last week of a three-week lactation showed that the embryo mortality that occurred by day 30 following mating of the restricted sows was largely a consequence of the death of female embryos (Vinsky et al. 2006). The weight and crown-rump length of surviving foetuses were lower in restricted fed sows, compared with foetuses from control-fed sows. Subsequent work from this group demonstrated that reduced embryonic development and survival of female embryos were associated with differences in the variance of epigenetic traits. Analysis of the variance in global DNA methylation and X-chromosome specific transcript (Xist) expression suggested that a sub-population of embryos within some litters from nutritionally restricted sows was epigenetically defective and lost before day 30 of gestation (Vinsky et al. 2007). Further evidence for nutritionally-induced skewing of conceptus gender comes from studies in which ewes receiving an enriched rumen-protected polyunsaturated fatty acid intake from four weeks prior to breeding and until day 13 post-oestrus had a higher proportion of male conceptuses (Green et al. 2008).
5. Longer-term effects
Transient alterations in nutrient supply during the peri-conception period can have long term and sometimes permanent effects on foetal development and the resultant offspring. These effects can be manifest at the level of the whole litter (effects on litter size, within-litter variability and sex ratios), in foetal or neonatal growth and in key organ systems and functions such as cardiovascular function, muscle biology, kidney development, reproductive potential, health and behaviour.
Examples of how pre- and peri-conception nutrition can affect a whole litter include observations that consumption of a high-fibre diet prior to mating increased litter size in pigs (Ferguson et al. 2004) while modifications to the diet such as supplementation with dextrose during the weaning to oestrus interval (van den Brand et al. 2006) or feeding diets that induced a modest reduction in circulating retinol concentrations during the first month of pregnancy (Antipatis et al. 2008) reduced within-litter variability in piglet birthweight.
At the level of the individual foetus or neonate, changes in key organ systems and function arising as a consequence of altered nutrient supply to the maternal body can arise in the absence of changes in foetal weight. The impact of altered nutrient supply on a range of foetal and neonatal endpoints has been the subject of considerable research effort and the topic of numerous reviews in recent years (see Watkins & Fleming (2009) and Ashworth et al. (2009) for details of programming of clinically and agriculturally important traits, respectively). This review will focus on the impact of altered nutrient supply during the peri-conceptual period and during embryonic life.
(a). Reproduction
Several recent reviews describe the effects of altered nutrient supply before conception and/or during pregnancy on the development of the foetal reproductive system and its subsequent function (Rhind 2004; Ashworth et al. 2005; Robinson et al. 2006; Gardner et al. 2008; Ashworth et al. 2009). Of particular note in the context of effects arising as a consequence of altered nutrition confined to the peri-conceptual or early pregnancy period are observations that feeding ewes 50 per cent energy requirements for live weight maintenance between days 0 and 30 of gestation resulted in foetal ovaries with more primordial follicles and fewer more developed primary and preantral follicles, when assessed on Day 110 of foetal life (Rae et al. 2001). This period of underfeeding could have affected only germ and somatic cells of the ovarian-mesonephros complex, as granulosa cells are not present at this stage of development in sheep foetuses. In the same experiment Rae et al. (2001) demonstrated that the onset of meiosis in ovarian germ cells was delayed in foetuses carried by mothers undernourished from mating until Day 65 of gestation, and that this effect was only evident if ewes had been undernourished throughout the period from Day 0 until day 65, rather than only from Day 30. These results indicate that the timing of the onset of meiosis may be mediated through effects on the germ cells, which in sheep foetuses are found in the gonad by about Day 35. Potential effects of alterations in the timing of meiosis on oocyte quality have not been assessed, although Kelly et al. (2005) found that oocytes collected from 9-week old ewe lambs born to ewes given sub-maintenance (0.7 × maintenance) diet from 71 to 110 and/or 101 to 126 days of gestation had a lower efficiency of in vitro blastocyst production than those from lambs of ewes that received 1.5 × maintenance during these periods.
Although undernutrition for at least the first 50 days of pregnancy affects the steroidogenic capacity of the foetal testis (Rae et al. 2002), there appear to be no effects on foetal testis development arising following undernutrition confined to the first 30 days of pregnancy.
(b). Cardiovascular effects
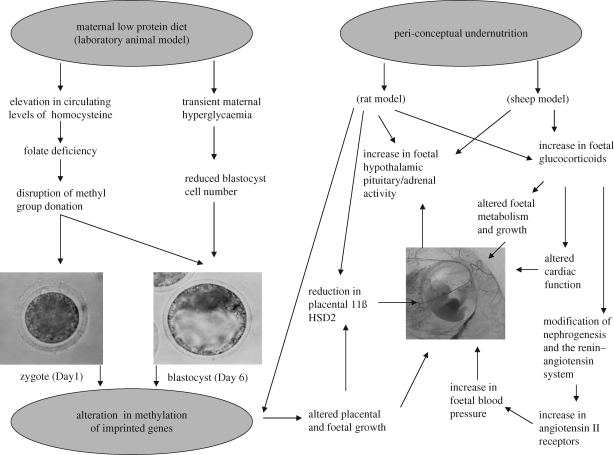
A series of studies conducted primarily in sheep or rodent models have demonstrated that relatively modest levels of undernutrition, when imposed during early pregnancy, elevate foetal, neonatal and adult blood pressure (reviewed by Ashworth et al. 2005). The possible mechanisms by which these changes could occur are shown diagrammatically in figure 1. In rodents, feeding a low protein diet exclusively during the pre-implantation stage of development has been shown to increase the expression of 11B-hydroxysteroid dehydrogenase type 1 (Hsd11b1) and phosphoenolpyruvate (Pepck, Pck1) genes involved in activating glucocorticoid in the foetal liver and gluconoegenesis, respectively, and induce relative hypertension in adult offspring. In sheep, maternal undernutrition from days 0 to 30 of pregnancy alters cardiovascular function of lambs at 1 year of age (Gardner et al. 2004), while in the adult, an increased interventricular septal wall thickness and increased mean left ventricular wall thickness were observed (Cleal et al. 2007). Maternal dietary deficiency in B vitamins and methionine of embryo donor ewes prior to embryo transfer on day 6 induced heavier, fatter and hypertensive offspring; these effects were most pronounced in males. These dietary changes were associated with widespread epigenetic alterations to DNA methylation in the offspring (Sinclair et al. 2007).
Figure 1.
Possible mechanisms for embryonic and early foetal programming of elevated blood pressure by maternal nutrition. Adapted from Ashworth et al. (2005).
(c). Behavioural effects
An intriguing observation to emerge from recent studies on developmental programming is that nutrition before conception and during the peri-conception period can alter the behaviour of the resultant offspring. For example, feeding a low protein diet (9% casein) to mice during the ovulatory cycle immediately preceding natural mating was associated with abnormal anxiety-related behaviour characterized by reduced exploratory behaviour, increased time spent resting and increased velocity in offspring of both sexes (Watkins et al. 2008). In sheep, sub-clinical cobalt deficiency in embryo donor ewes resulted in newborn lambs that spent less time interacting with their dams than lambs from embryos donated by cobalt-adequate ewes (Mitchell et al. 2007). Such observations confirm the importance of the nutritional status of the oocyte and/or early cleavage-stage embryo on post-natal behaviour.
6. Mechanisms
Given that the nutrient requirements of oocytes and embryos are minimal, it is perhaps surprising that changes in the composition or quantity of the diet consumed by the mother can have such profound and long-lasting effects on embryo, foetal and post-natal development. It is now widely accepted that changes in dietary intake promote changes in circulating concentrations of both metabolic hormones such as glucose, insulin, leptin and IGF-1 and reproductive hormones that in turn affect the developing ovarian follicle and/or the composition of reproductive tract secretions on which early embryos rely for their histotrophic nutrition. For example, using the experimental model whereby pigs were fed a high-fibre diet during the oestrous cycle preceding mating (referred to elsewhere in the review). Ferguson et al. (2007) presented data supporting a mechanistic endocrine link between increased dietary fibre prior to mating and increased prenatal survival. The working hypothesis proposed that the high-fibre diet promotes increased removal of circulating steroid, possibly by binding of steroid to fibre in the gut or modified bacterial enzyme activity and interrupted enterohepatic circulation of oestrogen. Lower circulating oestradiol concentrations would reduce the negative feedback effects of oestradiol on the hypothalamic pituitary axis, increasing the number of LH pulses and hence gonadotrophic support to the ovary, as reflected in the enhanced oocyte maturity observed. The way in which an altered LH profile influences oocyte maturity is not certain, but one possibility is that it could alter the composition of follicular fluid. Some studies describing nutritionally induced alterations in oocyte quality report a positive relationship between ooctye maturity and follicular fluid oestradiol concentrations (Yang et al. 2000; Ferguson et al. 2003), but this association was not observed in all studies and other follicular fluid components may also be important.
Throughout the last decade, there has been much interest in how environmental factors induce epigenetic modifications to oocytes and embryos which in turn contribute to altered developmental potential (Burdge et al. 2007). Imprinted genes, often showing allele-specific expression owing to epigenetic modification at regulatory CpG islands (differentially methylated regions) appear particularly sensitive to epigenetic changes. These changes are mediated by the pattern of DNA methylation and modifications to histone proteins which act in concert with chromatin structure to define the transcriptome associated with a specific cell lineage. Gametogenesis and early development are critical periods for the erasure, acquisition and maintenance of genomic imprints. A process of global demethylation in the primordial germ cells is followed by de novo methylation in the developing gonads. Methylation-dependent imprinting of the oocyte occurs post-natally, as antral follicles emerge and enter the process of follicular development. Methylation is complete by the metaphase II stage of oocyte nuclear maturation. In the immediate post-fertilization period in mammals, the methylated status of imprinted genes is maintained, whereas non-imprinted genes undergo a period of global demethylation and de novo methylation during this period.
Because the methylation imprint in the female is established at the time of oocyte maturation, the metabolic state of the female during the period of follicular growth preceding ovulation can exert important effects on the methylation process. For example, the data of Vinsky et al. (2007) show that restricted feeding of the sow during the last week of lactation was associated with reduced variance in DNA methylation in day 30 embryos. In addition, nutrition and metabolic state can affect methylation processes and hence global de novo methylation in early stages of embryo development (e.g. Kwong et al. 2006; Sinclair et al. 2007).
The role of DNA methylation and histone modifications in the regulation of monoallelic expression of imprinted genes is of particular significance to prenatal programming. For example, maternal expression of H19 in the female is paralleled by methylation-dependent suppression of paternal H19 as part of the imprinting process, whereas maternally expressed H19 and IGF2r are expressed but IGF2 is silenced. The interaction of these paternally and maternally expressed imprinted genes plays a critical role in determining the pattern of early embryonic development. As is evident from the preceding discussion, environmental perturbations that affect oocyte and/or embryo development alter expression of imprinted genes.
7. Consequences of pre-natal programming
The thrifty phenotype hypothesis (Hales & Barker 2001) proposes that the foetus responds to a poor (e.g. nutritionally poor) prenatal environment to increase the likelihood of its survival to term. The gene/proteome responds to such environmental cues, giving rise to post-natal phenotypes that are adapted to the adult environment predicted by the conditions of foetal life. However, although the offspring may be better adapted to a nutritionally poor postnatal environment it may be less well adapted to a more plentiful environment. Such a mismatch between predicted and actual post-natal environment has been implicated in health problems, particularly in societies where economic circumstances and nutrition are rapidly improving.
Much of the clinical literature describes foetal programming in a negative light, as ‘an an increased tendency towards a less-than-optimal phenotype’. However, the acute sensitivity of the developing oocyte and early embryo to environmental cues provides tremendous opportunities to programme desirable traits in offspring. Examples include the opportunity to increase the likelihood of prenatal survival, to improve muscle fibre characteristics in offspring and neonatal behaviour. Many of these attributes can be programmed by changes in the composition or amount of food consumed, which in turn, in livestock species, can contribute to required reductions in the environmental footprint of production systems.
8. Impact of altered diet and reproductive performance on the ‘environmental footprint’
While the environment in which the breeding female is reared, and particularly the environment immediately prior to conception and during the early stage of embryo development, can effect embryo development and the viability of the resultant offspring, reproductive efficiency can in turn affect the environment. Most studies to date have assessed the impact of improved reproductive efficiency on the environment in livestock species. This is because ruminant species make a significant contribution to greenhouse gas emissions. For example, recent estimates indicate that dairy cattle contribute about 20 per cent of total UK atmospheric methane emissions and 25 per cent of total UK ammonia emissions. Pig production was reported to account for 8.2 per cent of all agricultural greenhouse gas emissions in 2001, with the largest contribution coming from methane, owing to the high dependence on liquid manure storage (Janzen et al. 2006). Opportunities to reduce net greenhouse gas emissions from livestock production systems without compromising their productivity or the well-being of the animals themselves are the topic of intensive current research. This is clearly a complex and many-faceted area incorporating issues such as global trade, land use, fertilizer policy, crop choice and utilization, stock management systems and approach to slurry and manure storage. For the purposes of this review, the discussion will focus on how nutritionally-induced improvements in reproductive efficiency can contribute to reducing the environmental footprint.
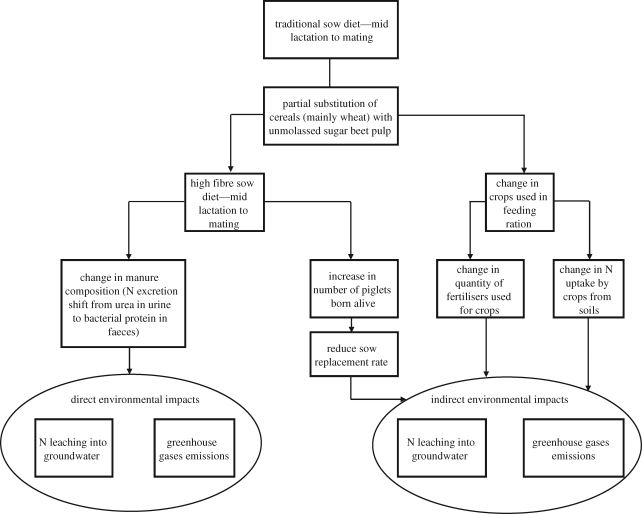
For example, dietary-induced improvements in litter size may reduce the environmental footprint of contemporary pig production systems both by reducing the number of sows required to produce a given amount of saleable pig meat and because the composition of the diet is recognized as a key means to reduce the environmental load from pig production (Aarnink & Verstegen 2007). Using a partial equilibrium model that included pig production and trade and environmental changes, Toma et al. (2008) predicted that the improvement in litter size following consumption of a diet containing unmolassed sugar beet pulp at an inclusion rate of 20 per cent during lactation and 40 per cent from weaning to oestrus (as described by Ferguson et al. 2004) would reduce the impact of such systems on the environment (air and groundwater pollution) by about 6 per cent (namely 6.34% for greenhouse gases emissions, methane and nitrous oxide in carbon equivalent, and 6.23% for nitrate losses through leaching/runoff into groundwater). The direct and indirect environmental impacts of this dietary change are shown diagrammatically in figure 2. Other studies describing the impact of increased dietary fibre in the form of sugar beet on pig systems also report reduced ammonia emissions (e.g. Canh et al. 1998; Fernandez et al. 1999; Clark et al. 2005). The overall effect on N balance depends on whether N was in surplus or deficit, because fibre reduces N digestibility but repartitions N from urine to faeces if in surplus (Morgan & Whittemore 1988) and on how the manure is stored or spread (Verge et al. 2009). The shift in nitrogen excretion from urea in urine to bacterial protein in faeces is a potential means to reduce the environmental load of pig production systems because the breakdown of protein in manure takes weeks or months while the degradation of urea to ammonia and CO2 occurs within several hours (Aarnink & Verstegen 2007). Such predictions would vary depending of the source of dietary fibre added, as this could affect the magnitude of the reproductive benefit, nitrogen excretion and methane production (Jorgensen 2007).
Figure 2.
Schematic diagram showing predicted direct and indirect environmental impacts of improved reproductive performance in the pig as a consequence of increased dietary fibre prior to mating.
Similar links between improvements in fertility and reduced greenhouse gas emissions have been described by Garnsworthy (2004). This report concludes that restoring dairy cattle fertility in the UK to levels typically found in the mid-1990s could reduce methane by 10–11% and ammonia emissions by 9 per cent and that, as with the case study in pigs described above, the use of nutritional strategies to improve fertility is likely to further reduce the environmental load.
A further dimension to the dynamic relationship between environmental change and reproductive efficiency is that a changing climate may in itself alter the availability and affordability of animal feedstuffs which affect reproductive performance. Examples include the increasing use of agricultural crops as bio-fuels (reviewed by Witzke et al. 2008) and the impact of climate change on appropriate varieties of crops for different geographical areas, disease and crop yield (e.g. Parry et al. 2004; Bouwman et al. 2006).
9. Conclusion
This review has highlighted the exquisite sensitivity of the developing oocyte and early embryo to changes in their immediate environment and to changes in the environment in which the female lives both before mating and during early pregnancy. Environmentally induced changes in oocyte and embryo biology have both immediate effects on the viability and development of these structures, but can also have pronounced and often persistent effects on the resultant offspring, including effects on the reproductive performance of successive generations. Sound nutritional management at key stages in the reproductive process provides an acceptable and effective way to improve reproductive outcome, not only in terms of the number of offspring born, but also in terms of their physiological well-being and viability. With increasing concern worldwide focused on climate change and food security it is crucial that nutritional strategies to improve reproductive outcome consider the environmental footprint of such developments and the predicted changes in feedstuff availability.
Footnotes
One contribution of 11 to a Theme Issue ‘Impacts of environmental change on reproduction and development in wildlife’.
References
- Aarnink A. J. A., Verstegen M. V. A.2007Nutrition, key factor to reduce environmental load from pig production. Livestock Sci. 109, 194–203 (doi:10.1016/j.livsci.2007.01.112) [Google Scholar]
- Adamiak S. J., Mackie K., Watt R. G., Webb R., Sinclair K. D.2005Impact of nutrition on oocyte quality: cumulative effects of body composition and diet leading to hyperinsulinemia in cattle. Biol. Reprod. 73, 918–926 (doi:10.1095/biolreprod.105.041483) [DOI] [PubMed] [Google Scholar]
- Adamiak S. J., Powell K., Rooke J. A., Webb R., Sinclair K. D.2006Body composition, dietary carbohydrates and fatty acids determine post-fertilisation development of bovine oocytes in vitro. Reproduction 131, 247–258 (doi:10.1530/rep.1.00871) [DOI] [PubMed] [Google Scholar]
- Al-Katanani Y. M., Webb D. W., Hansen P. J.1991Factors affecting seasonal variation in 90 day non-return rate to first service in lactating Holstein cows in a hot climate. J. Dairy Sci. 82, 2611–2615 [DOI] [PubMed] [Google Scholar]
- Al-Katanani Y. M., Paula-Lopes F. F., Hansen P. J.2002Effect of season and exposure to heat stress on oocyte competence in Holstein cows. J. Dairy Sci. 85, 390–396 [DOI] [PubMed] [Google Scholar]
- Antipatis C., Finch A. M., Ashworth C. J.2008Effect of controlled alterations in maternal dietary retinol on foetal and neonatal retinol status and pregnancy outcome in pigs. Livestock Sci. 118, 247–254 (doi:10.1016/j.livsci.2008.01.026) [Google Scholar]
- Ashworth C. J., Beattie L., Antipatis C., Vallet J. L.1999Effects of pre- and post-mating feed intake on blastocyst size, secretory function and glucose metabolism in Meishan gilts. Reprod. Fertil. Dev. 11, 323–327 (doi:10.1071/RD99040) [DOI] [PubMed] [Google Scholar]
- Ashworth C. J., McEvoy T. G., Rooke J. A., Robinson J. J.2005Nutritional programming of physiological systems throughout development. Trends Dev. Biol. 1, 117–129 [Google Scholar]
- Ashworth C. J., Ferguson E. M., Edwards S. A., Hunter M. G.2008Nutritional insights into the origins of embryonic loss in the pig. In Proc. of a Workshop on Embryonic and Fetal Nutrition, Ravello, Italy, May 2006, Havemeyer Foundation Monograph Series No. 21, pp. 27–29 Suffolk: R&W Communications [Google Scholar]
- Ashworth C. J., Dwyer C. M., McEvoy T. G., Rooke J. A., Robinson J. J.2009. The impact of in utero nutritional programming on small ruminant performances. In Nutritional and Foraging Ecology of Sheep and Goats. 12th Seminar of the FAO-CIHEAM Sub-Network on Sheep and Goat Nutrition, 11–13 October 2007, Thessaloniki, Greece (eds Papachristou T. G., Parissi Z. M., Ben Salem H., Morand-Fehr P.). Options Méditerranéennes (Series A: Mediterranean seminars Number 85), pp. 337–349 [Google Scholar]
- Benyei B., Gaspardy A., Cseh S.2003Effect of the El Nino phenomenon on the ovarian responsiveness and embryo production in donor cows. Acta Vet Hung 51, 209–218 (doi:10.1556/AVet.51.2003.2.9) [DOI] [PubMed] [Google Scholar]
- Bernardini C., Fantinati P., Zannoni A., Forni M., Tamanini C., Bacci M. L.2004Expression of HSP70/HSC70 in swine blastocysts: effects of oxidative and thermal stress. Mol. Reprod. Dev. 69, 303–307 (doi:10.1002/mrd.20143) [DOI] [PubMed] [Google Scholar]
- Borowczyk E., et al. 2006Effects of plane of nutrition on in vitro fertilisation and early embryonic development in sheep. J. Anim. Sci. 84, 1593–1599 [DOI] [PubMed] [Google Scholar]
- Biggers B. G., Geisert R. D., Wetteman R. P., Buchanan D. S.1987Effect of heat stress on early embryonic development in the beef cow. J. Anim. Sci. 64, 1512–1518 [DOI] [PubMed] [Google Scholar]
- Bouwman L., Van der Hoek K., Van Drecht G., Eickhout B.2006World livestock and crop production systems, land use and environment between 1970 and 2030. In Agriculture and climate beyond 2015—A New Perspective on Future Land Use Patterns (eds Brouwer F., McCarl B. A.). Dordrecht, The Netherlands: Springer [Google Scholar]
- Burdge G. C., Hanson M. A., Slater-Jeffries J. L., Lillycrop K. A.2007Epigenetic regulation of transcription: a mechanism for inducing variations in phenotype (foetal programming) by differences in nutrition during early life? Br. J. Nutr. 97, 1036–1046 (doi:10.1017/S0007114507682920) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canh T. T., Schrama J. W., Aranink A. J. A., Verstegen M. W. A., van Klooster C. E., Heetkamp M. J. W.1998Effect of dietary fermentable fibre from pressed sugar-beet pulp silage on ammonia emission from slurry of growing-finishing pigs. Anim. Sci. 67, 583–590 [Google Scholar]
- Clark O. G., Moehn S., Edeogu I., Price J., Leonard J.2005Manipulation of dietary protein and nonstarch polysaccharide to control swine manure emissions. J. Exp. Qual. 34, 1461–1466 (doi:10.2134/jeq2004.0434) [DOI] [PubMed] [Google Scholar]
- Cleal J. K., et al. 2007Mismatched pre- and postnatal nutrition leads to cardiovascular dysfunction and altered renal function in adulthood. Proc. Natl Acad. Sci. USA 104, 9529–9533 (doi:10.1073/pnas.0610373104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson E. M., Ashworth C. J., Edwards S. A., Hawkins N., Hepburn N., Hunter M. G.2003Effect of different nutritional regimens before ovulation on plasma concentrations of metabolic and reproductive hormones and oocyte maturation in gilts. Reproduction 126, 61–71 (doi:10.1530/rep.0.1260061) [DOI] [PubMed] [Google Scholar]
- Ferguson E. M., Ashworth C. J., Hunter M. G., Penny P., Slevin J., Edwards S. A.2004The effect of feeding a high fibre diet from mid lactation until breeding on subsequent litter size of sows. In The appliance of pig science (eds Thompson J. E., Gill B. P., Varley M. A.). BSAS Publication 31, pp. 175–179 Nottingham: Nottingham University Press [Google Scholar]
- Ferguson E. M., Slevin J., Hunter M. G., Edwards S. A., Ashworth C. J.2007Beneficial effects of a high fibre diet on oocyte maturity and embryo survival in gilts. Reproduction 133, 433–439 (doi:10.1530/REP-06-0018) [DOI] [PubMed] [Google Scholar]
- Fernandez J. A., Poulsen H. D., Boisen S., Rom H. B.1999Nitrogen and phosphorous consumption, utilisation and losses in pig production: Denmark. Livestock Prod. Sci. 58, 225–242 (doi:10.1016/S0301-6226(99)00011-1) [Google Scholar]
- Gardner D. S., Pearce S., Dandrea J., Walker R., Ramsay M. M., Stephenson T., Symonds M. E.2004Peri-implantation undernutrition programs blunted angiotensin II evoked baroreflex responses in young adult sheep. Hypertension 43, 1290–1296 (doi:10.1161/01.HYP.0000126991.67203.7b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner D. S., Lea R. G., Sinclair K. D.2008Developmental programming of reproduction and fertility: what is the evidence? Animal 2, 1128–1134 [DOI] [PubMed] [Google Scholar]
- Garnsworthy P. C.2004The environmental impact of fertility in dairy cows: a modelling approach to predict methane and ammonia emissions. Anim. Feed Sci. Technol. 112, 211–223 (doi:10.1016/j.anifeedsci.2003.10.011) [Google Scholar]
- Geisert R. D., Zavy M. T., Biggers B. G.1998Effect of heat stress on conceptus and uterine secretions in the bovine. Theriogenology 29, 1075–1082 (doi:10.1016/S0093-691X(88)80031-1) [DOI] [PubMed] [Google Scholar]
- Green M. P., et al. 2008Nutritional skewing of conceptus sex in sheep: effects of a maternal diet enriched in rumen-protected polyunsaturated fatty acids (PUFA). Reprod. Biol. Endocrinol. 9, 6–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C. N., Barker D. J.2001The thrifty phenotype hypothesis. Br. Med. Bull. 60, 5–20 (doi:10.1093/bmb/60.1.5) [DOI] [PubMed] [Google Scholar]
- Janzen H. H., et al. 2006A proposed approach to estimate and reduce net greenhouse gas emissions from whole farms. Can. J. Soil Sci. 86, 410–418 (doi:10.4141/S05-101) [Google Scholar]
- Jorgensen H.2007Methane emission by growing pigs and adult sows as influenced by fermentation. Livestock Sci. 109, 216–219 (doi:10.1016/j.livsci.2007.01.142) [Google Scholar]
- Kelly J. M., Kleeman D. O., Walker S. K.2005The effect of nutrition during pregnancy on the in vitro production of embryos from resulting lambs. Theriogenology 63, 2020–2031 (doi:10.1016/j.theriogenology.2004.09.007) [DOI] [PubMed] [Google Scholar]
- Kim M., Geum D., Khang I., Park Y. M., Kang B. M., Lee K. A., Kim K.2002Expression pattern of HSP25 in mouse preimplantation embryo: heat shock responses during oocyte maturation. Mol. Reprod. Dev. 61, 3–13 (doi:10.1002/mrd.1125) [DOI] [PubMed] [Google Scholar]
- Kwong W. Y., Miller D. J., Ursell E., Wild A. E., Wilkins A. P., Osmond C., Anthony F. W., Fleming T.2006Imprinted gene expression in the rat-embryo-fetal axis is altered in response to periconceptual maternal low protein diet. Reproduction 132, 265–267 (doi:10.1530/rep.1.01038) [DOI] [PubMed] [Google Scholar]
- Landau S., Bor A., Leibovich H., Zoref Z., Nitsan Z., Madar Z.1995The effect of rumen starch degradability in the diet of Booroola crossbred ewes on induced ovulation rate and prolificacy. Anim. Reprod. Sci. 38, 97–108 (doi:10.1016/0378-4320(94)01355-P) [Google Scholar]
- Martin G. B., Kadokawa H.2006‘Clean, green and ethical’ animal production. Case study: reproductive efficiency in small ruminants. J. Reprod. Dev. 52, 145–152 (doi:10.1262/jrd.17086-2) [DOI] [PubMed] [Google Scholar]
- Makarevich A. V., Olexikova L., Chrenek P., Kubovicova E., Freharova K., Pivko J.2007The effect of hyperthermia in vitro on vitality of rabbit preimplantation embryos. Physiol. Res. 56, 789–796 [DOI] [PubMed] [Google Scholar]
- McEvoy T. G., Robinson J. J., Aitken R. P., Findlay P. A., Robertson I. S.1997Dietary excesses of urea influence the viability and metabolism of preimplantation sheep embryos and may affect fetal growth among survivors. Anim. Reprod. Sci. 47, 71–90 (doi:10.1016/S0378-4320(96)01627-2) [DOI] [PubMed] [Google Scholar]
- Mitchell L. M., Robinson J. J., Watt R. G., McEvoy T. G., Ashworth C. J., Rooke J. A., Dwyer C. M.2007Effects of cobalt/vitamin B12 status in ewes on ovum development and lamb viability at birth. Reprod. Fertil. Dev. 19, 553–562 (doi:10.1071/RD07012) [DOI] [PubMed] [Google Scholar]
- Morgan C. A., Whittemore C. T.1988Dietary fibre and nitrogen excretion and retention by pigs. Anim. Feed Sci. Technol. 19, 185–189 (doi:10.1016/0377-8401(88)90066-1) [Google Scholar]
- Parry M. L., Rosenzweig C., Iglesias A., Livermore M., Fischer G.2004Effects of climate change on global food production under SRES emissions and socio-economic scenarios. Global Environ. Change 14, 53–67 [Google Scholar]
- Paula-Lopes F. F., Hansen P. J.2002Heat shock-induced apoptosis in preimplantation bovine embryos is a developmentally regulated phenomenon. Biol. Reprod. 66, 1169–1177 [DOI] [PubMed] [Google Scholar]
- Peters J. M., Wiley L. M., Zidenburg-Cherr S., Keen C. L.1991Influence of short-term maternal zinc deficiency on the in vitro development of preimplantation mouse embryos. Proc. Soc. Exp. Biol. Med. 198, 561–568 [DOI] [PubMed] [Google Scholar]
- Pisani L. F., Antonini S., Pocar P., Ferrari S., Brevini T. A., Rhind S. M., Gandolfi F.2008Effects of pre-mating nutrition on mRNA levels of developmentally relevant genes in sheep oocytes and granulosa cells. Reproduction 136, 303–312 (doi:10.1530/REP-07-0394) [DOI] [PubMed] [Google Scholar]
- Putney D. J., Drost M., Thatcher W. W.1988Embryonic development in superovulated dairy cattle exposed to elevated ambient temperatures between days 1 to 7 post insemination. Theriogenology 30, 195–209 (doi:10.1016/0093-691X(88)90169-0) [DOI] [PubMed] [Google Scholar]
- Rae M. T., Palassio S., Kyle C. E., Brooks A. N., Lea R. G., Miller D. W., Rhind S. M.2001Effect of maternal undernutrition during pregnancy on early ovarian development and subsequent follicular development in sheep fetuses. Reproduction 122, 915–922 (doi:10.1530/rep.0.1220915) [PubMed] [Google Scholar]
- Rae M. T., Rhind S. M., Fowler P. A., Miller D. W., Kyle C. E., Brooks A. N.2002Effect of maternal undernutrition on fetal testicular steroidogenesis during the CNS androgen-responsive period in male sheep fetuses. Reproduction 124, 33–39 (doi:10.1530/rep.0.1240033) [PubMed] [Google Scholar]
- Rhind S. M.2004Effect of maternal nutrition on fetal and neonatal reproductive development and function. Anim. Reprod. Sci. 82–83, 169–181 (doi:10.1016/j.anireprosci.2004.04.003) [DOI] [PubMed] [Google Scholar]
- Rivera R. M., Kelly K. L., Erdos G. W., Hansen P. J.2004Reorganisation of microfilaments and microtubules by thermal stress in two-cell bovine embryos. Biol. Reprod. 70, 1852–1862 (doi:10.1095/biolreprod.103.024901) [DOI] [PubMed] [Google Scholar]
- Robinson J. J., Rooke J. A., McEvoy T. G.2002Nutrition for conception and pregnancy. In Sheep nutrition (eds Freer M., Dove H.), pp. 189–211 Wallingford, UK: CAB International [Google Scholar]
- Robinson J. J., Ashworth C. J., Rooke J. A., Mitchell L. M., McEvoy T. G.2006Nutrition and Fertility in Ruminant Livestock. Anim. Feed Sci. Technol. 126, 259–276 (doi:10.1016/j.anifeedsci.2005.08.006) [Google Scholar]
- Rooke J. A., Ainslie A., Watt R. G., Alink F. M., McEvoy T. G., Sinclair K. D., Garnsworthy P. C., Webb R.2009Dietary carbohydrates and amino acids influence oocyte quality in heifers. Reprod. Fertil. Dev. 21, 419–427 (doi:10.1071/RD08193) [DOI] [PubMed] [Google Scholar]
- Roth Z.2008Heat stress, the follicle, and its enclosed oocyte: mechanism and potential strategies to improve fertility in dairy cows. Reprod. Domest. Anim. 43Suppl. 2, 238–244 [DOI] [PubMed] [Google Scholar]
- Schmidt S., et al. 2009Essential role of glucose transporter GLUT3 for post-implantation embryonic development. J. Endocrinol. 200, 23–33 (doi:10.1677/JOE-08-0262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair K. D., et al. 2007DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptual B vitamin and methionine status. Proc. Natl Acad. Sci. USA 104, 19 351–19 356 (doi:10.1073/pnas.0707258104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sviderskaya E. V., Jazrawi E., Baldwin S. A., Widnell C. C., Pasternak C. A.1996Cellular stress causes accumulation of the glucose transporter at the surface of cells independently of their insulin sensitivity. J. Membr. Biol. 149, 133–140 (doi:10.1007/s002329900014) [DOI] [PubMed] [Google Scholar]
- Toma L., Ashworth C. J., Stott A.2008. A partial equilibrium model of the linkages between animal welfare, trade and the environment in Scotland. In Proc., 109th EAAE Seminar The CAP after the Fischler reform: National Implementations, impact assessment and the agenda for future reforms, November 2008, Viterbo, Italy [Google Scholar]
- Van den Brand H., Soede N. M., Kemp B.2006Supplementation of dextrose to the diet during the weaning to estrus interval affects subsequent variation in within-litter piglet birthweight. Anim. Reprod. Sci. 91, 353–358 [DOI] [PubMed] [Google Scholar]
- Veras M. M., Damaceno-Rodrigues N. R., Silva R. M. G., Scoriza J. N., Saldiva P. H. N., Caldini E. G., Dolhnikoff M.2009Chronic exposure to fine particulate matter emitted by traffic affects reproductive and fetal outcomes in mice. Environ. Res. 109, 536–543 (doi:10.1016/j.envres.2009.03.006) [DOI] [PubMed] [Google Scholar]
- Verge X. P. C., Dyer J. A., Desjardins R. L., Worth D.2009Greenhouse gas emissions from the Canadian pork industry. Livestock Sci. 121, 92–101 (doi:10.1016/j.livsci.2008.05.022) [Google Scholar]
- Vinsky M. D., Novak S., Dixon W. T., Dyck M. K., Foxcroft G. R.2006Nutritional restriction in lactating primiparous sows selectively affects female embryo survival and overall litter development. Reprod. Fertil. Dev. 18, 347–355 (doi:10.1071/RD05142) [DOI] [PubMed] [Google Scholar]
- Vinsky M. D., Paradis F., Dixon W. T., Dyck M. K., Foxcroft G. R.2007Ontogeny of metabolic effects on embryonic development in lactating and weaned primaparous sows. Reprod. Fertil. Dev. 19, 603–611 (doi:10.1071/RD06116) [DOI] [PubMed] [Google Scholar]
- Wakefield S. L., Lane M., Schulz S. J., Hebart M. L., Thompson J. G., Mitchell M.2008Maternal supply of omega-3 polyunsaturated fatty acids alter mechanisms involved in oocyte and early embryo development in the mouse. Am. J. Physiol. Endocrinol. Metab. 294, E425–E434 (doi:10.1152/ajpendo.00409.2007) [DOI] [PubMed] [Google Scholar]
- Wang J.-Z., Shu H., Maio D.-Q., Liu N., Zhou P., Ge L., Tan H.2009Effects of heat stress during in vitro maturation on cytoplasmic versus nuclear components of mouse oocytes. Reproduction 137, 181–189 (doi:10.1530/REP-08-0339) [DOI] [PubMed] [Google Scholar]
- Watkins A. J., Fleming T. P.2009Blastocyst environment and its influence on offspring cardiovascular health: the heart of the matter. J. Anat. 215, 52–59 (doi:10.1111/j.1469-7580.2008.01033.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins A. J., et al. 2008Low protein diet fed exclusively during mouse oocyte maturation leads to behavioural and cardiovascular abnormalities in offspring. J. Physiol. 586, 2231–2244 (doi:10.1113/jphysiol.2007.149229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzke P., Banse M., Gomann H., Heckelei T., Breuer T., Mann S., Kempen M., Adenauer M., Zintl A.2008Modelling of energy crops in agricultural sector models—a review of existing methodologies JRC Scientific and Technical reports. Office for Official Publications of the European Communities, Luxembourg [Google Scholar]
- Yang H., Foxcroft G. R., Pettigrew J. E., Johnson L. J., Shurson G. C., Costa A. N., Zak L. J.2000Impact of dietary lysine intake during lactation on follicular development and oocyte maturation after weaning in primiparous sows. J. Anim. Sci. 78, 993–1000 [DOI] [PubMed] [Google Scholar]
- Zak L. J., Xu X., Hardin R. T., Foxcroft G. R.1997Impact of different patterns of feed intake during lactation in the primiparous sow on follicular development and oocyte maturation. J. Reprod. Fertil. 110, 99–106 [DOI] [PubMed] [Google Scholar]
- Zhu J. Q., Liu J. H., Liang X. W., Xu B. Z., Hou Y., Zhao X. X., Sun Q. Y.2008Heat stress causes aberrant DNA methylation of H19 and Igf-2r in mouse blastocysts. Mol. Cells 30, 211–215 [PubMed] [Google Scholar]