Abstract
Costs associated with extra-territorial movement are believed to have favoured the evolution of delayed dispersal and sociality across a range of social vertebrates, but remain surprisingly poorly understood. Here we reveal a novel mechanism that may contribute substantially to the costs of extra-territorial movement: physiological stress. We show that subordinate male meerkats, Suricata suricatta, exhibit markedly elevated faecal glucocorticoid metabolite levels (a non-invasive measure of hypothalamic–pituitary–adrenal axis activity) while conducting extra-territorial prospecting forays. While brief increases in glucocorticoid levels are unlikely to be costly, chronic elevations, arising from prolonged and/or frequent forays, are expected to compromise fitness through their diverse negative effects on health. Our findings strongly suggest that prolonged extra-territorial movements do result in chronic stress, as the high glucocorticoid levels of prospectors do not diminish on longer forays and are no lower among males with greater prospecting experience. A generalized ‘stress’ of extra-territorial movement may therefore have strengthened selection for delayed dispersal and sociality in this and other species, and favoured the conduct of brief forays over extended periods of floating. Our findings have implications too for understanding the rank-related distribution of physiological stress in animal societies, as extra-territorial movements are often conducted solely by subordinates.
Keywords: cooperative breeding, extra group, allostatic load
1. Introduction
In many social vertebrates, costs associated with extra-territorial movement are thought to have favoured the evolution of delayed dispersal, thereby setting the scene for the emergence of cooperative societies (Walters et al. 1992; Russell 2001). Despite their importance, such costs have rarely been demonstrated, due in part to the difficulty of monitoring individuals engaged in extra-territorial movements. A handful of studies do suggest that extra-territorial movements, whether forays or floating, can entail body condition loss or survival costs (e.g. Walters et al. 1992; Young et al. 2005; Ridley et al. 2008), although such comparisons can be complicated by confounds of floater/resident status (e.g. correlated variation in individual quality). The role of one factor that may contribute substantially to the costs of extra-territorial movement, however, remains entirely unexplored: physiological stress.
The stress response (activation of the hypothalamic–pituitary–adrenal axis, resulting in elevated glucocorticoid adrenal hormone secretion) is a generalized vertebrate response to environmental perturbations or threats (Balm 1999). While short-term ‘acute’ activation of the stress response is generally thought to aid survival (by releasing resources for ‘fight or flight’ responses), longer term ‘chronic’ stress can compromise fitness through its diverse negative effects on health (e.g. disruption of growth, reproduction, immunity; Balm 1999). As extra-territorial movements often necessitate travel alone through unfamiliar and/or poor-quality habitats coupled with the threat of aggression from predators and conspecifics, they might well be predicted to elicit acute stress in the short term and costly chronic stress when prolonged. Here we use non-invasive glucocorticoid monitoring to test this hypothesis in a cooperative carnivore, the meerkat, Suricata suricatta.
Meerkats are social mongooses that live in cohesive groups of 3–50 individuals that cooperatively defend their foraging home range against intrusion by neighbouring groups. The young of both sexes delay dispersal and help to rear the dominants' litters (Clutton-Brock et al. 1999). Subordinate males regularly conduct extra-territorial prospecting forays, for anything from a single afternoon to several months at a time, during which they approach foreign groups seeking reproductive and dispersal opportunities (Young et al. 2005, 2007; females do not prospect). Males typically lose body condition while prospecting and, at least in the short term, experience elevated circulating testosterone levels, due most likely to their aggressive interactions with foreign males and groups (Young et al. 2005). The effect that prospecting has on a male's stress physiology, however, has yet to be investigated. We ask whether: (i) subordinate males show elevated glucocorticoid levels while prospecting and (ii) such elevations are less pronounced among more experienced prospectors or those that have been away from their group for longer (which would reduce the likelihood and magnitude of chronic glucocorticoid elevations while prospecting). Finally, to establish whether our findings have implications too for the rank-related distribution of physiological stress (because only subordinate male meerkats prospect; Young et al. 2007), we compare the glucocorticoid levels of subordinate males within their groups with those of dominant males.
2. Material and methods
We studied nine free-ranging groups of individually identifiable meerkats of known life history, in the Kalahari Desert between 2000 and 2003 (Clutton-Brock et al. 1999; Young et al. 2005, 2007). All groups were habituated to close observation and visited at least once every 3 days to collect life-history data and faecal samples, and to weigh the animals on emergence from their sleeping burrows using an electronic balance. The dominant male in each group was behaviourally dominant to, and typically older and heavier than, all other males. Males were considered prospecting when sighted elsewhere (prospectors typically searched neighbouring territories and/or approached foreign groups; Young et al. 2007) or when absent from their own group (all group members typically forage, travel and sleep together; absent males were not known simply to forage elsewhere within the home range). Our analyses are restricted to adult males (above 1 year) as younger males rarely prospected. Whenever animals were seen defecating, faecal samples were collected, immediately placed on ice and then frozen on return to camp. All protocols conformed to the guidelines for the use of animals in research.
Steroid hormone metabolites were extracted from faeces and then assayed using a corticosterone radioimmunoassay kit (ICN Biomedicals) following methods previously validated for glucocorticoid metabolite determinations in meerkat faeces (Young et al. 2006). Assay sensitivity was 25 ng ml−1. Intra-assay coefficients of variation were less than 10 per cent and inter-assay coefficients of variation were 9.0 and 7.7 per cent. Faecal glucocorticoid (fGC) metabolite concentrations (ng g−1 dry faeces) were log transformed for analysis. Statistical analyses were conducted using GenStat 10 (Lawes Agricultural Trust, Harpenden, UK). Statistical model selection employed reverse stepwise elimination of fixed effects to yield a minimal model, whose structure was then confirmed using a forward stepwise approach. In all statistical models, repeated measures of groups and individuals were controlled using random factors, all two-way interactions were tested (none proved significant) and circadian variation in glucocorticoid excretion was controlled by fitting the hour of sample collection during the day as a fixed effect (in each case, a significant quadratic effect was identified, peaking in the middle of the day).
A general linear mixed model (GLMM) was used to investigate whether subordinate male fGC concentrations were higher while prospecting than while within their groups. This analysis used 264 faecal samples from 55 subordinate males from nine groups. In addition to whether the male was prospecting, the following fixed effects were fitted: male age (days); whether it was the peak conceptive season (June–January; Young et al. 2007); and sample collection hour. To allow for the possibility that considerable reductions in biomass intake (potentially associated with prospecting) could increase metabolite concentrations per unit faeces by reducing faecal matter throughput (Goymann et al. 2006), we also controlled for variation in males' recent net biomass intake. As glucocorticoid metabolites are pooled in the meerkat gut over 24–48 hours (Young et al. 2006), the male's change in body mass between the morning 2 days prior to sampling and the morning after sampling was used as the index of net biomass intake.
A second GLMM was used to investigate whether fGC concentrations while prospecting (107 samples from 36 males prospecting from eight groups) were lower in males with greater prospecting experience (number of forays previously conducted) or when males had been away from their group for longer (logarithm of the number of days since leaving; only forays with departure dates known to within a day were used). The male's age and net biomass intake were also fitted as fixed effects along with sample collection hour. As the majority of forays were conducted during the peak conceptive season, only samples collected during this time were used.
A third GLMM was used to investigate whether the fGC concentrations of subordinate males within their groups differed from those of dominant males (using 212 samples from 44 subordinates and 13 dominants from nine groups). In addition to the male's dominance status (dominant or subordinate), the following fixed effects were fitted: male age and net biomass intake; whether it was the peak conceptive season; and sample collection hour.
3. Results
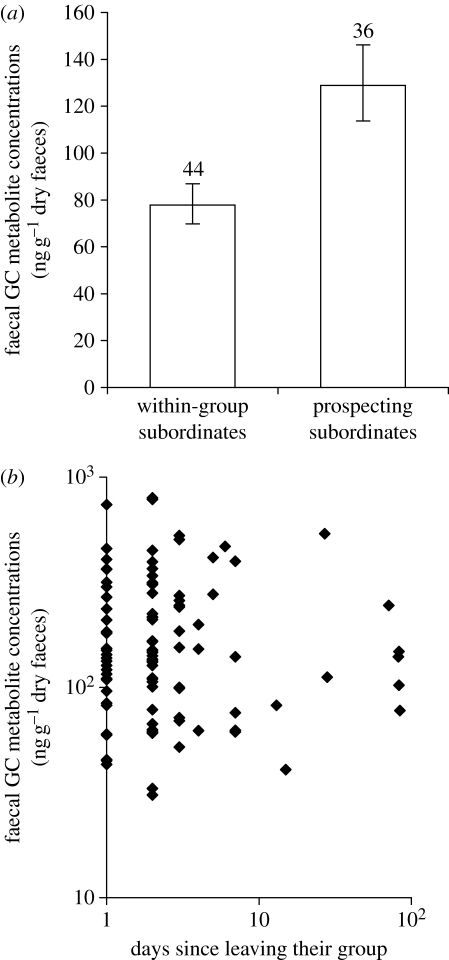
Subordinate adult males frequently conducted extra-territorial prospecting forays (up to 11 forays per month during the conceptive season, June–January (median 1, inter-quartile range (IQR) 0–3), for 1–150 days at a time (median 1, IQR 1–2.5)). The fGC concentrations of subordinate males were markedly higher while prospecting than while within their groups (GLMM: 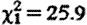 , p<0.001; figure 1
a), controlling for significant increases in fGC concentrations during the conceptive season (
, p<0.001; figure 1
a), controlling for significant increases in fGC concentrations during the conceptive season (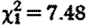 , p=0.007) and in older males (
, p=0.007) and in older males (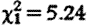 , p=0.026), and for sample collection timing effects (hour:
, p=0.026), and for sample collection timing effects (hour: 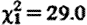 , p<0.001; hour2:
, p<0.001; hour2: 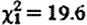 , p<0.001). Subordinate male fGC concentrations were not significantly higher when net biomass intake rates were lower (
, p<0.001). Subordinate male fGC concentrations were not significantly higher when net biomass intake rates were lower (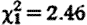 , p=0.13; the association trend was actually positive), and fGC concentrations while prospecting were still significantly elevated when net biomass intake rates were statistically controlled (
, p=0.13; the association trend was actually positive), and fGC concentrations while prospecting were still significantly elevated when net biomass intake rates were statistically controlled (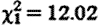 , p=0.001). Paired within-individual comparisons corroborate our principal finding: a sample of 22 subordinate males sampled in both conditions during the conceptive season showed higher median fGC concentrations while prospecting (median (IQR): 149.4 (100.6–213.1) ng g−1 dry faeces) than while within their groups (median (IQR): 88.9 (55.7–123.7) ng g−1 dry faeces; Wilcoxon signed-rank test statistic=51.0, n=22, p=0.013).
, p=0.001). Paired within-individual comparisons corroborate our principal finding: a sample of 22 subordinate males sampled in both conditions during the conceptive season showed higher median fGC concentrations while prospecting (median (IQR): 149.4 (100.6–213.1) ng g−1 dry faeces) than while within their groups (median (IQR): 88.9 (55.7–123.7) ng g−1 dry faeces; Wilcoxon signed-rank test statistic=51.0, n=22, p=0.013).
Figure 1.
(a) The fGC concentrations of subordinate males were markedly higher while extra-territorial prospecting than while within their groups (n=males). Bars represent means (±s.e.) after controlling for the other significant effects (§3). (b) Prospecting male fGC concentrations did not diminish with increasing time spent away from their groups (§3; the figure presents the raw data).
The elevated fGC concentrations of prospecting males did not diminish with increasing prospecting experience (GLMM: 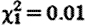 , p=0.97) or with increasing time spent away on a given foray (
, p=0.97) or with increasing time spent away on a given foray (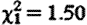 , p=0.22; figure 1
b), while controlling for sample collection timing effects (hour:
, p=0.22; figure 1
b), while controlling for sample collection timing effects (hour: 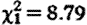 , p=0.004; hour2:
, p=0.004; hour2: 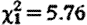 , p=0.018).
, p=0.018).
The fGC concentrations of subordinate males within their groups were comparable with those of dominant males (GLMM: 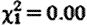 , p=0.95; means (±s.e.): subordinates 78.9 (±8.0); dominants 78.0 (±10.7) ng g−1 dry faeces), while controlling for fGC increases in older males (
, p=0.95; means (±s.e.): subordinates 78.9 (±8.0); dominants 78.0 (±10.7) ng g−1 dry faeces), while controlling for fGC increases in older males (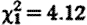 , p=0.046) and during the conceptive season (
, p=0.046) and during the conceptive season (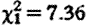 , p=0.007), and for sample collection timing effects (hour:
, p=0.007), and for sample collection timing effects (hour: 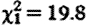 , p<0.001; hour2:
, p<0.001; hour2: 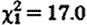 , p<0.001).
, p<0.001).
4. Discussion
Our findings reveal that subordinate male meerkats show markedly elevated fGC concentrations while extra-territorial prospecting, most probably reflecting stimulation of the physiological stress response (Balm 1999). This finding cannot be attributed to pre-existing physiological differences between prospectors and non-prospectors as it holds for paired comparisons within individuals. The substantial fGC elevations associated with prospecting are also unlikely to simply reflect reductions in biomass intake while prospecting (which might increase fGC concentrations by reducing faecal throughput), as they remain significant when net biomass intake rates are controlled. The apparent physiological ‘stress’ associated with prospecting most probably reflects the challenges that meerkat extra-territorial movements entail, including attacks by conspecifics (Young et al. 2005), elevated predation risk in the absence of cooperative vigilance (Clutton-Brock et al. 1999) and the associated disruption of foraging (Young et al. 2005). While glucocorticoid elevations are likely to be beneficial during brief forays (§1; Balm 1999), our finding that they do not diminish with increasing time spent away strongly suggests that prolonged extra-territorial movements (forays can last for more than three months) leave males subject to chronic stress and its associated fitness costs (Balm 1999).
As extra-territorial movements are likely to pose similar challenges for other social vertebrates, any associated physiological stress could prove a generalized mechanism through which costs of prolonged extra-territorial movement arise. We suggest that fitness costs arising from chronic stress may act in concert with, and indeed exacerbate, the more commonly invoked costs of extra-territorial movement (e.g. body condition loss or elevated mortality risk) to strengthen selection for delayed dispersal and favour brief extra-territorial forays over extended periods of floating. A generalized stress of extra-territorial movement would have important implications too for disease dynamics, as the immunosuppressive effects of chronic stress (Balm 1999) may render floaters and dispersers differentially susceptible vectors for disease.
As extra-group movements are often conducted solely by subordinates (see Young et al. 2005, 2007 and references therein), our findings have implications too for understanding the rank-related distribution of physiological stress in animal societies (Creel 2001; Goymann & Wingfield 2004). Our comparison of the average glucocorticoid levels of dominant and subordinate males within their groups (a comparison commonly employed to investigate the rank-related distribution of physiological stress) suggests that the two classes experience comparable levels of stress. However, as only subordinate males prospect, the stress experienced by subordinates may markedly exceed that of dominants during peak prospecting periods. Attempts to quantify the rank-related distribution of physiological stress within animal societies should therefore seek, whenever possible, to integrate the full range of ecological contexts faced by each focal class.
Acknowledgements
All protocols conformed to the guidelines for the use of animals in research. The research was approved by the University of Pretoria Research Ethics Committee.
We thank the Mammal Research Institute, University of Pretoria and our volunteers their for valuable assistance, Tim Clutton-Brock for access to the study population and research supervision, and the Natural Environment Research Council, Magdalene College, Cambridge and the Smithsonian Institution for funding.
References
- Balm P.H.M.Stress physiology in animals. 1999Sheffield, UK:Sheffield Academic Press [Google Scholar]
- Clutton-Brock T.H, Gaynor D, McIlrath G.M, Maccoll A.D.C, Kansky R, Chadwick P, Manser M, Skinner J.D, Brotherton P.N.M.1999Predation, group size and mortality in a cooperative mongoose. Suricata suricatta J. Anim. Ecol 68, 672–683doi:10.1046/j.1365-2656.1999.00317.x [Google Scholar]
- Creel S.2001Social dominance and stress hormones. Trends Ecol. Evol 16, 491–497doi:10.1016/S0169-5347(01)02227-3 [Google Scholar]
- Goymann W, Wingfield J.C.2004Allostatic load, social status and stress hormones: the costs of social status matter. Anim. Behav 67, 591–602doi:10.1016/j.anbehav.2003.08.007 [Google Scholar]
- Goymann W, Trappschuh M, Jensen W, Schwabl I.2006Low ambient temperature increases food intake and dropping production, leading to incorrect estimates of hormone metabolite concentrations in European stonechats. Horm. Behav 49, 644–653doi:10.1016/j.yhbeh.2005.12.006 [DOI] [PubMed] [Google Scholar]
- Ridley A.R, Raihani N.J, Nelson-Flower M.J.2008The cost of being alone: the fate of floaters in a population of cooperatively breeding pied babblers Turdoides bicolor. J. Avian Biol 39, 389–392doi:10.1111/j.0908-8857.2008.04479.x [Google Scholar]
- Russell A.F.2001Dispersal costs set the scene for helping in an atypical avian cooperative breeder. Proc. R. Soc. B 268, 95–99doi:10.1098/rspb.2000.1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters J.R, Doerr P.D, Carter J.H.1992Delayed dispersal and reproduction as a life-history tactic in cooperative breeders—fitness calculations from red-cockaded woodpeckers. Am. Nat 139, 623–643 [Google Scholar]
- Young A.J, Carlson A.A, Clutton-Brock T.2005Trade-offs between extraterritorial prospecting and helping in a cooperative mammal. Anim. Behav 70, 829–837doi:10.1016/j.anbehav.2005.01.019 [Google Scholar]
- Young A.J, Carlson A.A, Monfort S.L, Russell A.F, Bennett N.C, Clutton-Brock T.2006Stress and the suppression of subordinate reproduction in cooperatively breeding meerkats. Proc. Natl Acad. Sci. USA 103, 12005–12010doi:10.1073/pnas.0510038103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A.J, Spong G, Clutton-Brock T.2007Subordinate male meerkats prospect for extra-group paternity: alternative reproductive tactics in a cooperative mammal. Proc. R. Soc. B 274, 1603–1609doi:10.1098/rspb.2007.0316 [DOI] [PMC free article] [PubMed] [Google Scholar]