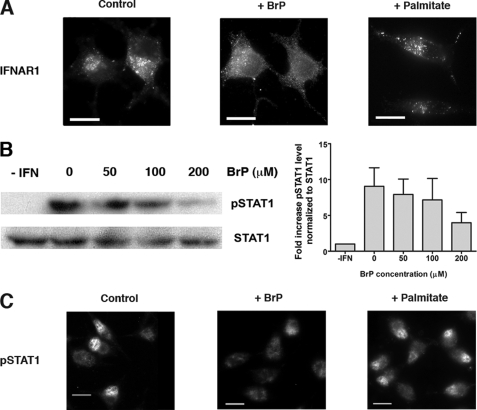
FIGURE 1.
IFNAR1 endocytosis and IFN-α-induced Jak/Stat signaling depend on palmitoylation events. A, effect of the palmitoylation inhibitor 2-bromopalmitate (BrP)on IFNAR1 endocytosis. L929R1R2 cells, pretreated or not for 1 h with 200 μm 2-bromopalmitate or 200 μm palmitate, were incubated with anti-IFNAR1 34F10 mAb as described under “Experimental Procedures.” Cells were incubated at 37 °C for 30 min with 1000 units/ml IFN-α2b, and IFNAR1 endocytosis was detected by visualizing internalized IFNAR1-antibody complexes after an acid wash. Scale bar, 20 μm. Results are representative of at least four independent experiments. B, effect of 2-bromopalmitate on IFN-α-induced Stat1 tyrosine phosphorylation. L929R1R2 cells were pretreated for 1 h with the indicated concentrations of 2-bromopalmitate and stimulated with 1000 units/ml IFN-α for 10 min at 37 °C. Total lysates were analyzed by Western blot/ECL to detect Stat1 and phosphorylated Stat1 (pStat1). Quantification of pStat1 phosphorylation was made using ImageJ software, and the results correspond to the ratio between pStat1 amount and tubulin amount normalized to the control without 2-bromopalmitate. Results represent mean and S.D. of three independent experiments. C, effect of 2-bromopalmitate on IFN-α-induced pStat1 nuclear translocation. L929R1R2 cells were pretreated or not for 1 h with 200 μm 2-bromopalmitate or 200 μm palmitate and stimulated with 1000 units/ml IFN-α2b for 30 min at 37 °C. Cells were fixed, and the nuclear localization of phosphorylated Stat1 was detected by immunofluorescence. Results are representative of at least three independent experiments. Scale bar, 20 μm.