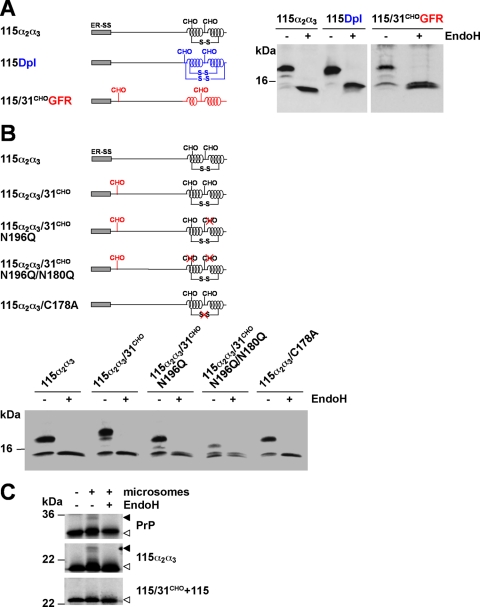
FIGURE 3.
ER import of unstructured domains is restored by increasing the content in α-helical domains. A and B, schematic presentation of the mutants analyzed. S-S, disulfide bond. The following domains were fused to PrP-115 or PrP-115/31CHO: 115α2α3, two α-helical domains of PrP (black); 115Dpl, two α-helical domains of Doppel (blue); and 115/31CHOGFR, two α-helical domains of GFR (red). A and B, N2a cells were transiently transfected with the mutants depicted. Cell lysates were either treated with EndoH (+ EndoH) or left untreated (− EndoH) and analyzed by Western blot using the mAb 3F4. C, α-helical but not unstructured domains restore in vitro translocation. PrP, 115α2α3, and 115/31CHO+115 were synthesized in vitro in the presence (+ microsomes) or absence (− microsomes) of ER-derived rough microsomes. If indicated, radioactively labeled products were treated with EndoH (± EndoH) before SDS-PAGE. Open arrowheads, unglycosylated PrP species; closed arrowheads, glycosylated PrP species.