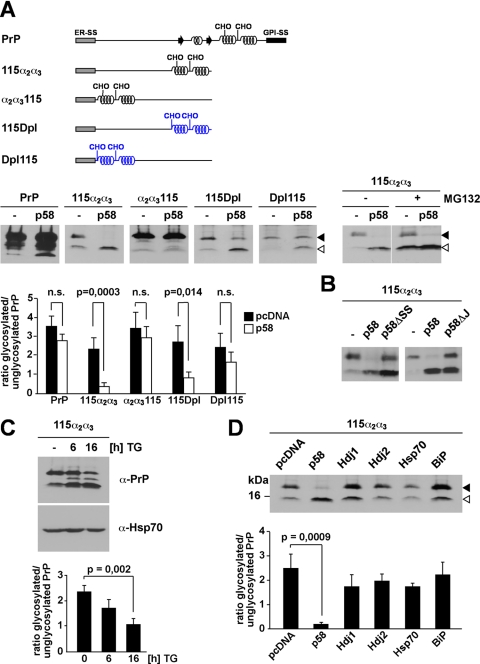
FIGURE 5.
p58IPK promotes proteasomal degradation of ER-targeted polypeptides with extended unstructured domains at their N terminus. A, p58IPK promotes a preemptive/co-translocational quality control pathway. N2a cells were transiently co-transfected with the constructs indicated and p58IPK (p58) or a vector control (−). Right panel, cells co-transfected with p58IPK and PrP-115α2α3 were incubated in the presence or absence of MG132 (30 μm; 3 h). The lanes MG132+ are positioned directly next to the lanes MG132− although all lanes originate from one gel. B, p58IPK requires the ER signal peptide and J-domain to interfere with ER import of PrP-115α2α3. N2a cells were transiently co-transfected with PrP-115α2α3 and either p58IPK (p58), p58IPKΔSS (p58ΔSS), p58IPKΔJ (p58ΔJ), or a vector control (−). A and B, expression of PrP was analyzed by immunoblotting using the mAb 3F4; an open arrowhead marks the unglycosylated protein species; a closed arrowhead marks the glycosylated forms. C, reduced import under conditions of ER stress. N2a cells transfected with PrP-115α2α3 were incubated with thapsigargin (TG; 1 μm) for the time indicated and then analyzed by Western blot using the mAb 3F4 for detection of PrP and anti-Hsp70 mAb for detection of endogenous Hsp70 as loading control. D, overexpression of cytosolic Hsp70 or BiP does not interfere with ER import. N2a cells were transiently co-transfected with PrP-115α2α3 and the constructs indicated. Expression of PrP-115α2α3 was then analyzed by immunoblotting using the mAb 3F4; an open arrowhead marks the unglycosylated PrP species; a closed arrowhead marks the glycosylated forms. A, B, and C, quantifications were based on at least three independent experiments. Data were expressed as the ratio of glycosylated versus unglycosylated PrP (mean ± S.E.). Statistical analysis was performed using Student's t test. n.s., not significant.