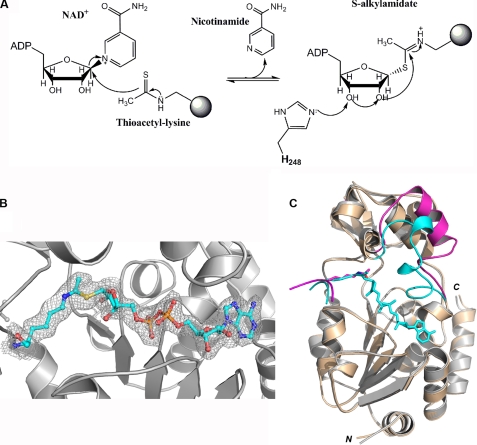
FIGURE 5.
Structure of the SIRT3-AceCS2-Ks-ac crystal with a 1-h NAD+ soak (SIRT3-AceCS2-Ks-ac-ADPR). A, the initial step of dethioacetylation reaction by SIRT3. The NAM moiety of NAD+ is released first followed by the ADPR moiety of NAD+ transferred to the thioacetyl lysine, generating the S-alkylamidate. B, the Fo − Fo omit electron density map (1 σ) for the S-alkylamidate intermediate (in stick representation) is presented by gray wires. C, superimposition of the SIRT3-AceCS2-Kac and SIRT3-AceCS2-Ks-ac-ADPR structures. The regions that have similar conformations are colored in gray for the SIRT3-AceCS2-Kac structure and in tinted yellow for the SIRT3-AceCS2-Ks-ac-ADPR structure. The flexible-loop region that has significant conformational difference is highlighted in magenta for the SIRT3-AceCS2-Kac structure and in blue for the SIRT3-AceCS2-Ks-ac-ADPR structure.