FIGURE 8.
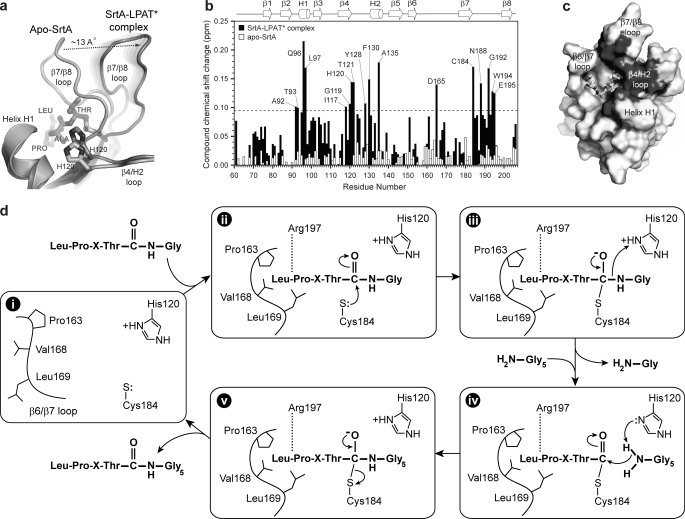
Localization of the lipid II binding site and proposed mechanism of transpeptidation. a, superposition of the NMR structures of apo-SrtAΔN59 (34) (Protein Data Bank code 1ija) and the SrtAΔN59-LPAT* complex. The image shows an expanded view of the substrate-dependent structural change in the β7/β8 loop that unmasks a groove leading into the active site. The image was generated in a similar manner as the one shown in Fig. 3b. b, histogram plot of the compound chemical shift changes for the backbone amide hydrogen and nitrogen atoms of SrtAΔN59 after the addition of the Gly3 tripeptide. Chemical shift changes after the addition of the peptide to either the SrtAΔN59-LPAT* complex (black) or SrtAΔN59 in its apo-state (white) are shown. The secondary structure of the enzyme and amino acids experiencing the largest changes are labeled. The data indicate that only SrtA in the context of the SrtAΔN59-LPAT* complex binds to the Gly3 tripeptide. The dashed line represents one S.D. above the average chemical shift perturbation of all amino acids. c, solvent-accessible surface of SrtAΔN59 in the complex with residues that are significantly perturbed by the addition of the Gly3 peptide colored dark gray. The majority of the chemical shift changes occur near the active site around the surface uncovered when the β7/β8 is displaced from helix H1. d, transpeptidation mechanism based on the structure of the SrtAΔN59-LPAT* complex and biochemical data. i, apoenzyme containing a flexible β6/β7 loop; ii, sorting signal binding closes the β6/β7 loop. Arg197 hydrogen-bonds to the backbone, and the threonine carbonyl carbon is attacked by the thiolate of Cys184. iii, the displaced β7/β8 loop serves as an exit point for residues COOH-terminal to the LPXTG motif. This enables His120 to protonate the amide of the glycine residue as the scissile bond is broken. iv, the cross-bridge peptide of lipid II enters the active site via the surface unmasked by the movement of the β7/β8 loop. v, a second tetrahedral intermediate forms, followed by the rupture of the Cys184-sorting signal linkage and product release.