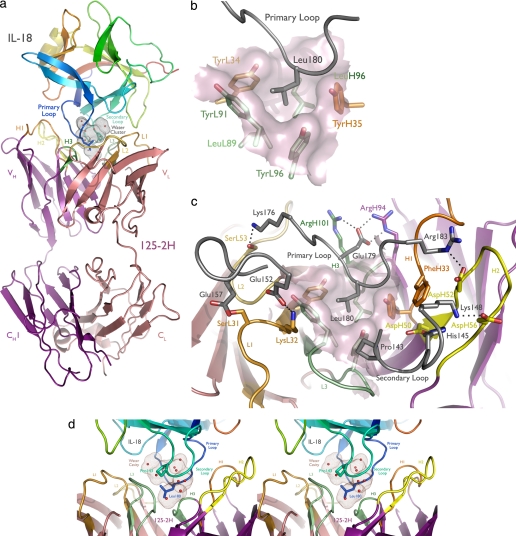
FIGURE 2.
125-2H binds human IL-18 residue Leu180 in a deep pocket, trapping 10 water molecules. a, overview of the complex. The antibody engages the primary (Leu180) and secondary (Pro143) IL-18 loops. IL-18 is colored as a rainbow, from the NH2 to the COOH terminus; CDRs 1, 2, and 3 of the 125-2H Fab fragment (purple, heavy chain; pink, light chain) are colored orange, yellow, and green, with the heavy chain in darker tones. The solvent-inaccessible, water-filled cavity trapped between 125-2H Fab and IL-18 is shown (brown dots). b, the center of the combining site, viewed from the perspective of IL-18 (gray). Note the deep hydrophobic pocket, formed by heavy and light chain Tyr and Leu residues, that binds Leu180. c, the periphery of the combining site is ringed by charge-charge and hydrogen bonding interactions involving all six 125-2H CDRs. d, stereoview illustrating the large cavity (brown dots) formed between the IL-18 primary and secondary loops and the 125-2H CDRs, trapping 10 well ordered water molecules. The detailed hydrogen bond interactions are shown in supplemental Fig. 2.