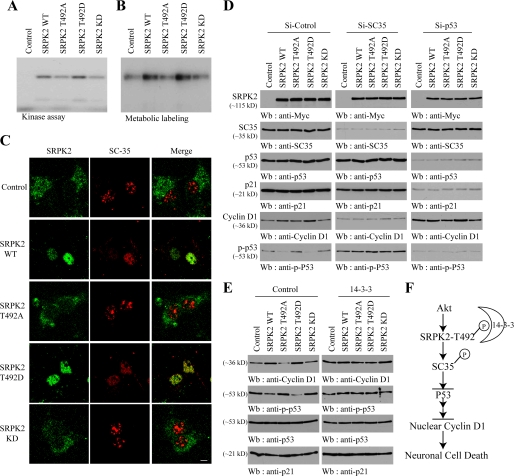
FIGURE 7.
SRPK2 phosphorylates SC35, inhibits p53 activation, and up-regulates cyclin D1. A, in vitro SRPK2 kinase assay with SC35 recombinant protein. The reaction mixture was resolved on 10% SDS-PAGE. Wild-type SRPK2 and T492D mutant strongly phosphorylated SC35, but T492A and KD mutants were unable to phosphorylate it. B, SRPK2 phosphorylates SC35 in neurons. Various SRPK2 lentivirus were infected into cortical neurons and followed by metabolic labeling in [32P]H3PO4. SC35 was pulled down and resolved on SDS-PAGE. Wild-type SRPK2 and T492D mutant strongly phosphorylated SC35, whereas other mutants displayed weak effect. C, SRPK2 redistributes SC35 in the nucleus. Cortical neurons were infected with various SRPK2 lentivirus. Localization of various SRPK2 and endogenous SC35 was visualized by indirect immunofluorescent staining using anti-Myc polyclonal antibody and anti-SC35 antibody. Wild-type SRPK2 and T492D mutant strongly redistributed SC35 from the nuclear speckles into the nucleoplasm. D, depletion of SC35 abolishes SRPK2-regulated cyclin D1 expression. Various SRPK2 constructs co-transfected with si-control, si-SC35, or si-p53 into cortical neurons. The lysates were analyzed by Western blotting using antibodies against indicated proteins. E, 14-3-3 inhibits the SRPK2 effect on p53 activation. Various SRPK2 constructs were co-infected with control and 14-3-3 into cortical neurons. The lysates were analyzed by immunoblotting with various antibodies. Wild-type SRPK2 and T492D repressed p53 phosphorylation and increased cyclin D1 expression, which was abolished by 14-3-3. F, a schematic model for Akt/SRPK2/sc35/p53/cyclin D1 signaling in neuronal cell cycle and cell death.