Abstract
Distinguishing primary ovarian carcinoma from metastatic carcinoma to the ovary is often difficult by histologic examination alone. Recently an immunohistochemical marker CDX-2 was found to be of considerable diagnostic value in establishing the gastrointestinal origin of metastatic tumors. The aim of this study was to determine whether CDX-2 can distinguish between these malignancies. Paraffin-embedded tissue sections from 57 primary ovarian tumors and 40 metastatic tumors to the ovary were immunostained for CDX-2, and results were compared to the ancillary immunohistochemical results for CK7/CK20, CEA, CA125, and her-2/neu. CDX-2 immunoreactivity was observed in most of metastatic carcinomas with colorectal (91%) and appendiceal (100%) origin, however CDX-2 was negative in all primary ovarian carcinomas, except for the mucinous subtype. Almost all primary ovarian carcinomas including the mucinous subtype showed diffuse and strong immunoexpression for CK7. CEA and CA125 were mainly found in metastatic and primary ovarian carcinoma, respectively. Her-2/neu overexpression was only noted in a small proportion of primary and metastatic ovarian carcinomas. These results suggest that CDX-2 is very useful immunohistochemical marker for distinguishing metastatic colorectal carcinoma to the ovary from primary ovarian carcinoma, including the mucinous subtype. Furthermore, combination with CDX-2 and CK7 strengthen the differential diagnosis between these tumors.
Keywords: Cdx-2-3 protein, Ovarian Neoplasms, Adenocarcinoma, Neoplasm Metastasis, Immunohistochemistry
INTRODUCTION
In terms of metastasis, the ovaries are the most commonly involved organ, in the female genital tract. The appearance of ovaries containing metastases vary greatly, and the primary ovarian tumors are a heterogeneous groups of neoplasms, with different histopathologic features and variable clinical behaviors. It is known that findings suggestive of metastasis are bilaterality, the presence of multiple discrete nodules either within the substance of the ovary or on its surface, and vascular invasion (1). However, a significant proportion of metastatic carcinomas to the ovary show histologic features reminiscent of primary ovarian carcinoma, and in some instances, histologic features show considerable overlap (2). Thus it may be difficult to differentiate between these two entities by using clinical and histologic backgrounds, but the differential diagnosis is of paramount importance for therapeutic and prognostic purposes. Immunohistochemistry may play a fundamental role in this circumstance. Currently available immunohistochemical markers, such as cytokeratin (CK)7, CK20, carcinoembryonic antigen (CEA), human alveolar macrophage-56 (HAM-56), mucin marker (MUC)2, and MUC5AC, are of limited sensitivity and specificity, even though in many settings, they allow the site of origin of a metastatic lesion to be identified (3-7). For this reason, continued interest is shown in the development of new and more specific markers for intestinal differentiation. CDX-2, a homeobox gene encoding an intestine-specific transcription factor, appears to be such a marker (8). CDX-2 plays a role in directing early processes in intestinal cell morphogenesis and in the maintenance of the differentiated phenotype by supporting the transcription of differentiated gene products. Moreover, recent studies have shown that CDX-2 has a specific expression in normal colonic epithelium and in most colorectal adenocarcinomas, and thus CDX-2 immunohistochemistry has been suggested to be of use for establishing the gastrointestinal origins of metastatic tumors (9), although the expression of CDX-2 is not entirely exclusive to intestinal carcinomas (10). In this study, we examined CDX-2 expression in all subtypes of primary ovarian adenocarcinoma and in metastatic adenocarcinoma to the ovary, including metastasis of gastric and appendiceal origin. We also evaluated the usefulness of an immunohistochemical panel consisting of CK7, CK20, CEA, CA125, and her-2/neu, for distinghuising between these two groups of lesions. The aim of this study was to investigate the possible immunohistochemical use of CDX-2 in this context, and to identify a combination of immunohistochemical markers that can be used for the differential diagnosis of primary ovarian carcinoma and metastatic carcinoma.
MATERIALS AND METHODS
Materials
Primary and metastatic ovarian carcinoma samples were obtained from the Department of Pathology of Yeungnam University Hospital. The series included 26 cases of serous adenocarcinoma, 18 cases of mucinous adenocarcinoma, 6 cases of endometrioid adenocarcinoma, 7 cases of clear cell adenocarcinoma, and 40 cases of metastatic adenocarcinoma of different origin including 18 stomach, 11 colorectum, 7 appendix, 1 breast, 1 lung, 1 pancreas, and 1 kidney. Appendiceal metastases were low-grade mucinous epithelial tumors. Specimens were obtained by surgical resection in all cases. All metastatic carcinomas had recognized original sites proven by histologic examination of the primary lesion. The distinction between primary and metastatic mucinous adenocarcinomas was sometimes based on well-defined clinicopathologic criteria (11).
Tissue microarray
For immunohistochemical analysis a tissue microarray (TMA) technique was used, which allowed the staining of a large numbers of specimens on one slide. TMAs were prepared manually using a dermal punch biopsy needle, reconstructed by our laboratory. Details of the technique have been described previously (12). To account for cancer tissue heterogeneity, 2 to 3 cylindrical core biopsies, 2.0 mm in diameter, were carefully taken from different sites of each tumor to include all histological patterns, and then arrayed in a recipient paraffin TMA block.
Immunohistochemistry
Sections (4 µm) were mounted on poly-L-lysine-coated slides for immunohistochemical analysis using a labeled streptavidin-biotin-peroxidase method (DAKO, Carpinteria, CA, U.S.A.). Primary antibodies to CDX-2, CK7, CK20, CEA, CA125, and her-2/neu were using in the study (Table 1). Briefly, TMA sections were deparaffinized, rehydrated in graded alcohols and treated for 5 min with 1% H2O2. Sections for CA125, and her-2/neu immunohistochemistry were subjected to microwave antigen retrieval and then incubated for 30 min. After rinsing sections in PBS and incubating them with normal goat serum for 6 min, all pre-diluted primary antibodies were applied for 60 min at room temperature. The sections were then allowed to react with peroxidase conjugated streptavidin for 45 min. Color development was performed using diaminobenzidine, and then counterstained with hematoxylin. Colorectal mucosa and adenocarcinomas were used as positive controls for CDX-2. Immunoreactiviy was considered positive if more than 10% of the neoplastic cells showed nuclear (CDX-2, her-2/neu) or cytoplasmic (CK7/20, CEA, CA125) immunoreactivity. Tumors were scored in a semiquantitative method according to the following scheme: 0 (less than 10% of tumor cells); 1+ (positive signal of any intensity in 11-40% of tumor cells); 2+ (greater than 41% of tumor cells).
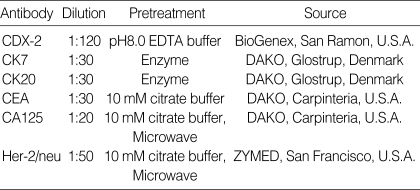
Table 1.
Antibodies used in this study
Statistical analysis
Immunohistochemical data were expressed as total numbers (or percentages) of cases showing positive staining. The relations between primary ovarian tumors and metastatic tumors, with or without immunoreactivity were evaluated using the chi-square test. Statistical significance was for p values <0.05. The specificity and sensitivity of the investigated markers for primary and metastatic ovarian carcinomas were calculated as follows: specificity=true negatives/(true negatives+false positives); and sensitivity=true positives/(true positives+false negatives).
RESULTS
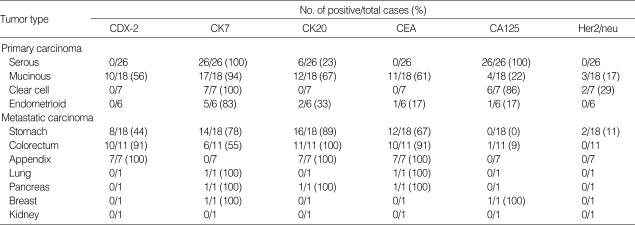
The results of the immunohistochemical studies are summarized in Table 2.
Table 2.
Immunohistochemical results in evaluated ovarian tumors
CDX-2-immunohistochemical findings
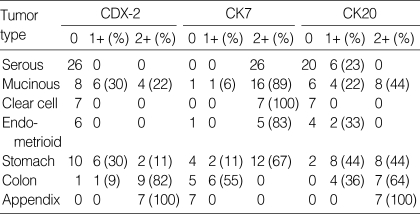
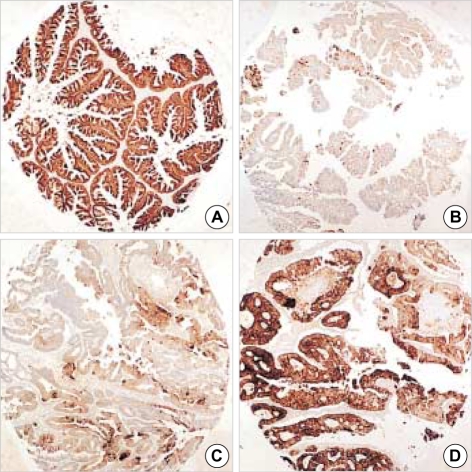
CDX-2 immunoreactivity was visualized as a fine granular to diffuse staining pattern confined to nuclei. Control specimens showed that most nuclei of the normal intestinal epithelium and in colorectal adenocarcinoma cells were strongly reactive for CDX-2. Overall, CDX-2 immunoreactivity was detected in 10 of 57 (18%) primary ovarian carcinomas, and 25 of 40 (63%) metastatic ovarian carcinomas, with significance (p<0.0001). In primary ovarian carcinoma, CDX-2 immunostaining was observed in 10 of 18 (56%) cases of mucinous adenocarcinoma only, and all serous, endometrioid, and clear cell adenocarcinomas of the ovary were CDX-2 negative. In metastases, CDX-2 expression was identified in 7 of 7 (100%) of appendiceal origin, 10 of 11 (91%) of colorectal origin, and in 8 of 18 (44%) of gastric origin. All cases of colorectal and appendiceal origin were strongly and diffusely positive (2+) for CDX-2, which compared with weak, focal positivity (1+) in other immunoreactive tumors (Table 3, Fig. 1). CDX-2 appears to be a highly sensitive and specific immunologic marker of intestinal differentiation. No CDX-2 immunostaining was identified in 4 cases of metastatic adenocarcinomas from lung, pancreas, breast, or kidney.
Table 3.
The expression of CDX-2, CK7, CK20 in primary and metastatic ovarian tumors according to the extent of positivity
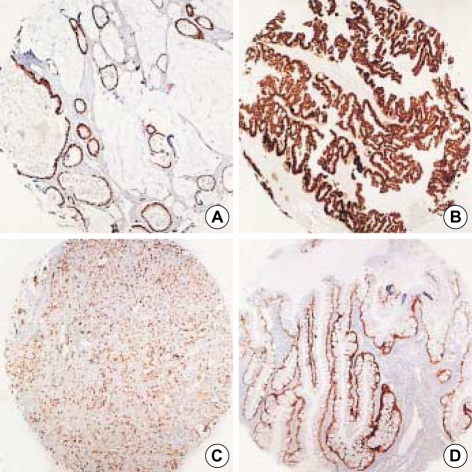
Fig. 1.
Representative sections of diffuse CDX-2 immunoreactivity in primary ovarian mucinous adenocarcinoma (A) and in metastatic carcinoma from colorectum (B), stomach (C) and appendix (D) (×40).
CK7- and CK20-immunohistochemical findings
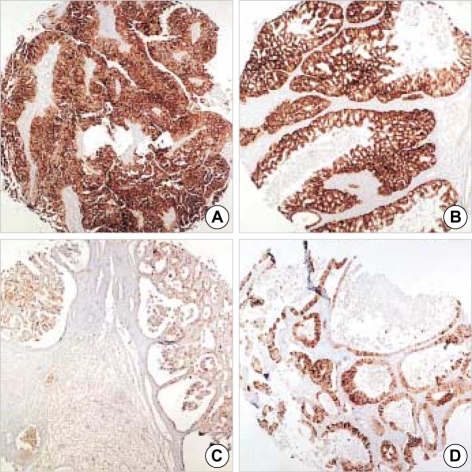
CK7 and CK20 immunoreactivities were observed in 55 of 57 (97%) and 20 of 57 (35%) primary ovarian carcinomas, respectively, and in 23 of 40 (58%) and 35 of 40 (88%) metastatic carcinomas. CK7 was expressed in all serous subtypes and in 17 of 18 (94%) of mucinous subtype, whereas CK20 expression was observed in all cases (100%) of colorectal and appendiceal carcinoma, and in 16 of 18 (89%) cases of gastric carcinoma. A tendency was observed for more diffuse CK7 staining in primary ovarian carcinoma rather than in metastatic carcinoma and conversely, the expression of CK20 was more likely to be diffuse in metastases than in primaries (Table 3, Fig. 2). The immunoprofiles of CK7 and CK20 were significantly different in these two groups (p<0.0001).
Fig. 2.
Almost all primary ovarian mucinous carcinomas are diffusely positive for CK7 (A), and variably positive for CK20, often showing patchy rather than diffuse staining (B). Conversely, metastatic colorectal carcinoma had a focal CK7 (C) and diffuse CK20 (D) immunoprofile (×40).
CEA-immunohistochemical findings
CEA expression revealed a significant differences between primary versus metastatic tumors in the ovary (p<0.0001). CEA staining was noted in 11 of 18 (61%) of ovarian mucinous carcinomas, and in 10 of 11 (91%) metastatic carcinomas of colorectal origin and in all of appendiceal origin. CEA immunoreactivity was prevalent in gastrointestinal tumors (Table 4).
Table 4.
The expression of CEA, CA125, Her-2/neu in primary and metastatic ovarian tumors according to the extent of positivity
CA125-immunohistochemical findings
CA125 was expressed in 35 of 57 (61%) and in 2 of 40 (5%) primary and metastatic tumors, respectively, with significance (p<0.0001). In particular, the serous subtype showed 100% reactivity for CA125 (Fig. 3), whereas only 4 of 18 (22%) of the mucinous subtype showed CA125 immunostaining.
Fig. 3.
Diffuse strong cytoplasmic staining for CA125 is observed in ovarian serous carcinoma (A). Her-2/neu expression is evident in a small group of the ovarian mucinous (B) and clear cell carcinomas (C) and unexpectidly in metastatic carcinomas from the stomach (D) (×40).
Her-2/neu-immunohistochemical findings
No significant difference was observed between the primary and metastatic groups with respect to her-2/neu expression. Her-2/neu immunoreactivity was observed in 2 of 7 (29%) primary clear cell subtype, 3 of 18 (17%) mucinous subtype, and in 2 of 18 (11%) metastatic gastric carcinomas (Fig. 3).
DISCUSSION
Metastatic carcinomas account for approximately 15% of malignant neoplasms involving the ovary. The most common primary sites are the stomach, large bowel, appendix, breast, uterus, lung and skin. In this study, metastases of gastric origin was most frequent, probably due to the exceptional prevalence of gastric carcinoma in Korea. In contrast, metastatic carcinoma from the breast showed a very low incidence, even though breast carcinoma is now the most common malignancy in Korean women. Moreover 80% of ovarian metastases from extragenital sites are derived from gastrointestinal tract carcinomas and these commonly mimic primary neoplasms (2). The problems encountered in attempting to differentiate between primary ovarian carcinoma and ovarian metastases from colonic carcinoma on a histological basis alone have prompted an intensive search for immunohistochemical markers that could aid this differential diagnosis. A growing body of literature (13-16) has recently dealt with CDX-2 immunoreactivity in benign and malignant lesions of the gastrointestinal tract, such as intestinal metaplasia of the stomach, intestinal-type gastric cancer, and primary and metastatic intestinal adenocarcinomas. Our analysis concurs with other studies with respect to CDX-2 immunostaining results (10, 17-19). We found a high prevalence of CDX-2 immunoreactivity, which showed a more homogeneous, stronger staining patterns, in metastatic carcinomas from colon and appendix. However CDX-2, either focal or diffuse, was also expressed in more than 50% of primary mucinous carcinomas. CDX-2 was not expressed by non-mucinous subtypes, which contrasts to the 20% and 10.5% immunoreactivity for CDX-2 observed in endometrioid carcinomas as reported by Logani et al. (18) and Kaimaktchiev et al. (20). In addition, significant CDX-2 expression in ovarian mucinous adenocarcinoma has been demonstrated by other series, in which it was observed in from 10.5% to 100% of cases (14, 17-19). The result of the present study might be of great diagnostic value with respect to the differentiation of primary non-mucinous adenocarcinomas and metastatic colorectal carcinomas, but as in many settings, this marker can not distinguish primary mucinous carcinoma from metastatic mucinous carcinoma to the ovary. Fraggetta et al. (19) suggested that CDX-2 should be considered as a cell-type related antigen rather than as an organ specific marker. Extra-intestinal immunoreactivity for CDX-2 has been rarely reported in urinary bladder adenocarcinoma, thyroid papillary carcinoma, carcinoid tumor (10), lung adenocarcinomas (21), cervical adenocarcinoma (22), and sinonasal adenocarcinoma (23) as well as in ovarian mucinous lesions (10, 19), although CDX-2 immunoreactivity showed, by far, the highest prevalence in carcinomas of intestinal origin. This unexpected expression of CDX-2 needs further investigation.
The combined expression profiles of CK7 and CK20, the most widely used immunohistochemical markers, is already an established parameter in the differential diagnosis of ovarian primaries from metastatic carcinoma to the ovary. The coordinate expression of these markers has been very useful for distinguishing between ovarian carcinomas (usually CK7+/CK20-) and colorectal carcinomas (usually CK7-/CK20+) (3, 4). However, occasionally colorectal carcinoma shows CK7 expression and conversely, CK20 expression has been observed in primary ovarian carcinoma (24, 25), and thus this immunohistochemical staining is not helpful for differential diagnoses. The data presented in this study and in other reports (3, 4) indicate that almost all primary ovarian carcinomas are diffusely positive for CK7 and variably positive for CK20, and conversely, that almost all metastatic colorectal carcinomas are diffusely positive for CK20 and variably positive for CK7. Therefore, considerations of staining distributions of CK7 and CK20 within a tumor still have a great value in terms of identifying the primary site of a metastatic lesion. In particular, the markers used in this study have diagnostic utility in terms of excluding metastatic colorectal adenocarcinomas from ovarian mucinous and endometrioid adenocarcinomas, which most commonly mimic intestinal adenocarcinoma.
CEA expression appears to offer another route of approaching the differential diagnosis of primary and metastatic lesions. CEA is overexpressed in a variety of human tumors, including cervical, colorectal, gastric, pancreatic, ovarian, breast, and non-small cell lung carcinomas (27). In our series, CEA was mainly expressed in carcinomas of colorectal and appendiceal origin, and in ovarian carcinoma, especially of the mucinous subtype. This study therefore indicates that CEA is not suitable for differentiating ovarian mucinous carcinoma from metastatic colorectal carcinoma, as shown by the data presented in CDX-2 (Table 2). However CDX-2 was more superior to CEA because CEA was less specific to establish intestinal differentiation in that CEA immunoreactivity was more prevalent in primary mucinous carcinoma, compared to CDX-2, and also overexpressed in metastatic pulmonary and pancreatic carcinoma. In this situation, a combined panel of CEA and other known markers, such as CK7 and CK20, is necessary to distinguish primary and metastatic mucinous carcinomas. CA125 is undoubtedly associated with primary ovarian carcinoma (28). CA125 immunoreactivity was found to be a good marker for identifying serous and clear cell subtypes, regardless of differentiation. Thus CA125 immunoreactivity could be useful for the subtyping of ovarian carcinoma. However it is not helpful at distinguishing ovarian mucinous carcinomas from metastatic mucinous carcinomas, even though the latter showed a tendency of focal staining. The overexpression of her-2/neu is observed in 25-30% of breast carcinomas and this is associated with a poor prognosis. Relatively little is known concerning the relationship between her-2/neu status and ovarian carcinoma (29). In a few instances her-2/neu immunoreactivity was detected in ovarian clear cell carcinomas (29, 30). This marker was demonstrated in 29% of clear cell carcinoma and 17% of mucinous carcinoma, according to this study, and interestingly her-2/neu immunoreactivity was found to be present in 2 cases of metastatic gastric carcinoma. However at present, the literature contains no data on her-2/neu expression in intestinal carcinoma.
Taken together the results available to date suggest that the differential diagnosis of primary and metastatic mucinous carcinoma pose greatest difficulty because both tumors can share an immunophenotype and gross and microscopic features. CDX-2 immunoreactivity may help in their differential diagnosis, but this marker is not entirely restricted to cells showing the morphological appearance of intestinal type epithelium, and is rarely aberrantly expressed in other tumor cells. Therefore, CDX-2 immunoreactivity should be supported by an immunohistochemical panel of well-known markers. We conclude that CDX-2 is a highly sensitive marker for colonic carcinoma metastatic to the ovary, and that CDX-2 and CK7 are the best combination of immunohistochemical marker for differentiating metastatic carcinoma to the ovary from primary ovarian carcinoma including the mucinous subtype.
Footnotes
This research was supported by a Yeungnam University research grants in 2004.
References
- 1.Scully RE, Young RH, Philip BC. Atlas of tumor pathology: Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament. Washington D.C: AFIP; 1998. (3rd series). [Google Scholar]
- 2.Daya D, Nazerali L, Frank GL. Metastatic ovarian carcinoma of large intestinal origin simulating primary ovarian carcinoma. A clinicopathologic study of 25 cases. Am J Clin Pathol. 1992;97:751–758. doi: 10.1093/ajcp/97.6.751. [DOI] [PubMed] [Google Scholar]
- 3.Ueda G, Sawada M, Ogawa H, Tanizawa O, Tsujimoto M. Immunohistochemical study of cytokeratin-7 for the differential diagnosis of adenocarcinomas in the ovary. Gynecol Oncol. 1993;51:219–223. doi: 10.1006/gyno.1993.1276. [DOI] [PubMed] [Google Scholar]
- 4.Loy TS, Calaluce RD, Keeney GL. Cytokeratin immunostaining in differentiating primary ovarian carcinoma from metastatic colonic adenocarcinoma. Mod Pathol. 1996;9:1040–1044. [PubMed] [Google Scholar]
- 5.Berezowski K, Stastny JF, Kornstein MJ. Cytokeratin 7 and 20 and carcinoembryonic antigen in ovarian and colonic carcinoma. Mod Pathol. 1996;9:426–429. [PubMed] [Google Scholar]
- 6.Fowler LJ, Maygarden SJ, Novotny DB. Human alveolar macrophage-56 and carcinoembryonic antigen monoclonal antibdies in the differential diagnosis between primary ovarian and metastatic gastrointestinal carcinomas. Hum Pathol. 1994;25:666–670. doi: 10.1016/0046-8177(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 7.Albarracin CT, Jafri J, Montag AG, Hart J, Kuan SF. Differential expression of MUC2 and MUC5AC mucin genes in primary ovarian and metastatic colonic carcinoma. Hum Pathol. 2000;31:672–677. doi: 10.1053/hupa.2000.6799. [DOI] [PubMed] [Google Scholar]
- 8.Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol. 1996;16:619–625. doi: 10.1128/mcb.16.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seno H, Oshima M, Taniguchi MA, Usami K, Ishikawa TO, Chiba T, Taketo MM. CDX2 expression in the stomach with intestinal metaplasia and intestinal-type cancer: prognostic implications. Int J Oncol. 2002;21:769–774. doi: 10.3892/ijo.21.4.769. [DOI] [PubMed] [Google Scholar]
- 10.Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303–310. doi: 10.1097/00000478-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Lee KR, Young RH. The distinction between primary and metastatic mucinous carcinomas of the ovary: gross and histologic findings in 50 cases. Am J Surg Pathol. 2003;27:281–292. doi: 10.1097/00000478-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 13.Bai YQ, Yamamoto H, Akiyama Y, Tanaka H, Takizawa T, Kake M, Kenji Yagi O, Saitoh K, Takeshita K, Iwai T, Yuasa Y. Ectopic expression of homeodomain protein CDX2 in intestinal metaplasia and carcinomas of the stomach. Cancer Lett. 2002;176:47–55. doi: 10.1016/s0304-3835(01)00753-4. [DOI] [PubMed] [Google Scholar]
- 14.Li MK, Folpe AL. CDX-2, a new marker for adenocarcinom of gastrointestinal origin. Adv Anat Pathol. 2004;11:101–105. doi: 10.1097/00125480-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Eda A, Osawa H, Yanaka I, Satoh K, Mutoh H, Kihira K, Sugano K. Expression of homeobox gene CDX2 precedes that of CDX1 during the progression of intestinal metaplasia. J Gastroenterol. 2002;37:94–100. doi: 10.1007/s005350200002. [DOI] [PubMed] [Google Scholar]
- 16.Vassil K, Luig T, Luig T. The homeobox intestinal differentiation factor CDX2 is selectively expressed in gastrointestinal adenocarcinomas. Mod Pathol. 2004;17:1392–1399. doi: 10.1038/modpathol.3800205. [DOI] [PubMed] [Google Scholar]
- 17.Groisman GM, Meir A, Sabo E. The value of CDX2 immunostaining in differentiating primary ovarian carcinomas from colonic carcinomas metastatic to the ovaries. Int J Gynecol Pathol. 2004;23:52–57. doi: 10.1097/01.pgp.0000101141.31270.a0. [DOI] [PubMed] [Google Scholar]
- 18.Logani S, Oliva E, Arnell PM, Amin MB, Young RH. Use of novel immunohistochemical markers expressed in colonic adenocarcinoma to distinguish primary ovarian tumors from metastatic colorectal carcinoma. Mod Pathol. 2005;18:19–25. doi: 10.1038/modpathol.3800260. [DOI] [PubMed] [Google Scholar]
- 19.Fraggetta F, Peloci G, Cafici A, Nuciforo P, Viale G. CDX2 immunoreactivity in primary and metastatic ovarian mucinous tumors. Virchows Arch. 2003;443:782–786. doi: 10.1007/s00428-003-0910-3. [DOI] [PubMed] [Google Scholar]
- 20.Kaimaktchieve V, Terracciano L, Tornillo L, Spichtin H, Stoios D, Bundi M, Korcheva V, Mirlacher M, Loda M, Sauter G, Corless CL. The homeobox intestinal differentiation factor CDX 2 is selectively expressed in gastrointestinal adenocarcinomas. Mod Pathol. 2004;17:1392–1399. doi: 10.1038/modpathol.3800205. [DOI] [PubMed] [Google Scholar]
- 21.Barbareschi M, Murer B, Colby TV, Chilosi M, Macri E, Loda M, Doglioni C. CDX-2 homeobox gene expression is a reliable marker of colorectal adenocarcinoma metastatses to the lungs. Am J Surg Pathol. 2003;27:141–149. doi: 10.1097/00000478-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Raspollini MR, Baroni G, Taddei A, Taddei GL. Primary cervical adenocarcinoma with intestinal differentiation and colonic carcinoma metastatic to cervix. An investigation using CDX-2 and a limited immunohistochemical panel. Arch Pathol Lab Med. 2003;127:1586–1590. doi: 10.5858/2003-127-1586-PCAWID. [DOI] [PubMed] [Google Scholar]
- 23.Franchi A, Massi D, Palomba A, Biancalani M, Santucci M. CDX-2, cytokeratin 7 and cytokeratin 20 immunohistochemical expression in the differential diagnosis of primary adenocarcinomas of the sinonasal tract. Virchows Arch. 2004;445:63–67. doi: 10.1007/s00428-004-1030-4. [DOI] [PubMed] [Google Scholar]
- 24.McCluggage WG. Recent advances in immunohistochemistry in the diagnosis of ovarian neoplasm. J Clin Pathol. 2000;53:327–334. doi: 10.1136/jcp.53.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagendijk JH, Mullink H, Van Diest PJ, Meijer GA, Meijer CJ. Tracing the origin of adenocarcinomas with unknown primary using immunohistochemistry: differential diagnosis between colonic and ovarian carcinomas as primary sites. Hum Pathol. 1998;29:491–497. doi: 10.1016/s0046-8177(98)90065-x. [DOI] [PubMed] [Google Scholar]
- 26.Wauters CC, Smedts F, Gerrits LG, Bosman FT, Ramaekers FC. Keratins 7 and 20 as a dignostic markers of carcinomas metastatic to the ovary. Hum Pathol. 1995;26:852–855. doi: 10.1016/0046-8177(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 27.Lash RH, Hart WR. Intestinal adenocarcinomas metastatic to the ovaries. A clinicopathologic evaluation of 22 cases. Am J Surg Pathol. 1987;11:114–121. doi: 10.1097/00000478-198702000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Lagendijk JH, Mullink H, van Diest PJ, Meijer GA, Meijer CJ. Immunohistochemical differentiation between primary adenocarcinomas of the ovary and ovarian metastases of colonic and breast origin. Comparison between a statistical and an intuitive approach. J Clin Pathol. 1999;52:283–290. doi: 10.1136/jcp.52.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cirisano FD, Karlan BY. The role of the HER-2/neu oncogene in gynecologic cancers. J Soc Gynecol Invest. 1996;3:99–105. [PubMed] [Google Scholar]
- 30.Iwamoto H, Fukasawa H, Honda T, Hirata S, Hoshi K. HER-2/neu expression in ovarian clear cell carcinomas. Int J Gynecol Cancer. 2003;13:28–31. doi: 10.1046/j.1525-1438.2003.13028.x. [DOI] [PubMed] [Google Scholar]