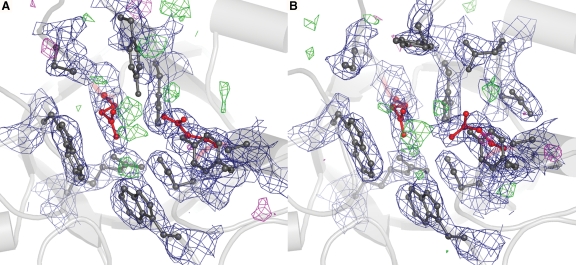
Fig. 3.
Electron density around the catalytic center. Catalytic residues E235 and E340 are shown as red balls and sticks and surrounding residues are in dark gray. Contours of the 2Fo–Fc map are shown as a blue mesh (at 1.2σ); contours of the Fo–Fc map are shown in green mesh (at 3σ) and in magenta (at −3σ). Several Fo–Fc peaks are visible in the active site, but they did not overlap with the 2Fo–Fc map, nor are they continuous; hence, at this resolution they appear to be noise. A and B show the catalytic centers of molecules A and B, respectively, in the asymmetric unit.