Figure 4.
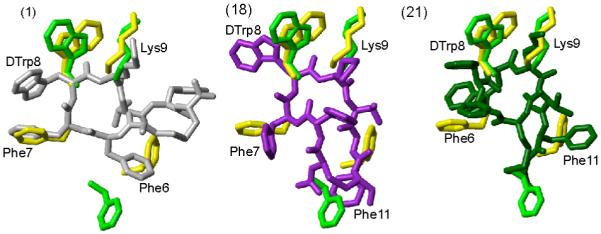
Superposition of receptor-specific pharmacophore with the 3D NMR structure of the analogues 1, 18 and 21. The sst2 pharmacophore41 is shown in green. The octreotide pharmacophore proposed by Melacini et al.44 is shown in yellow. In both the pharmacophores only the side chains of the amino acids are shown that are involved in binding to the receptor. For analogues 1, 18 and 21, the conformer with the lowest energy is used to represent the 3D structures. The analogues are color coded as in Figure 2. The side chains of the amino acids, which are proposed to be involved in receptor binding, are labeled. Phe6 in analogue 1, Phe11 in analogues 18 and 21 should undergo a change in their conformation to fit either the sst2 or octreotide pharmacophores.
