Table 1.
Screen of Michael Acceptors
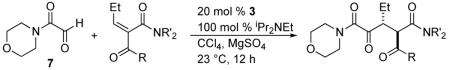
| entry | R | R' | product | yield (%)a | ee (%)b | drc |
|---|---|---|---|---|---|---|
| 1 | Me | Me | 8 | 68 | 82 | 6:1 |
| 2 | Et | 9 | 66 | 77 | 14:1 | |
| 3 | Ph | Me | 10 | 60 | 7 | 14:1 |
| 4 | Et | Me | 11 | 92 | 89 | 5:1 |
| 5d | Me | 11 | 90 | 92 | 12:1 | |
Reaction conducted with 1 equiv of 7 and 2 equiv of Michael acceptor at 23 °C.
Enantiomeric excess determined by HPLC analysis on a chiral stationary phase.
Diastereomer ratio determined by 1H NMR.
Reaction conducted at 0 °C.
