Abstract
Background:
The death of an attachment figure triggers intrusive thoughts of the deceased, sadness, and yearning for reunion. Recovery requires reduction of symptoms. We hypothesized that symptoms might correlate with a capacity to regulate attention toward reminders of the deceased, and activity in, and functional connectivity between, prefrontal regulatory regions and the amygdala.
Methods:
Twenty recently bereaved subjects rated intrusive thoughts of the deceased versus a capacity to avoid thoughts (grief style). Reaction time was measured while subjects completed an Emotional Stroop (ES) task contrasting deceased-related with control words during functional magnetic resonance imaging (fMRI). Subjects subsequently visualized the death of the deceased and rated induced emotions.
Results:
Subjects demonstrated attentional bias toward deceased-related words. Bias magnitude correlated with amygdala, insula, dorsolateral prefrontal cortex (DLPFC) activity. Amygdala activity predicted induced sadness intensity. A double dissociation between grief style and both prefrontal and amygdala subregion activity was found. Intrusiveness correlated with activation of ventral amygdala and rostral anterior cingulate (rACC); avoidance correlated with deactivation of dorsal amygdala and DLPFC. A double dissociation between regulatory region and task-dependent functional connectivity (FC) was found. High DLPFC-amygdala FC correlated with reduced attentional bias, while low rACC-amygdala FC predicted sadness intensity.
Conclusions:
Results are consistent with a model in which activity in and functional connectivity between the amygdala and prefrontal regulatory regions indexes differences in mourners' regulation of attention and sadness during pangs of grief, and may be used to distinguish between clinically relevant differences in grief style.
Keywords: Attention, bereavement, cognitive control, emotion regulation, incentive salience, separation distress
A central task of bereavement (1-3) is to reduce the frequency and intensity of three cardinal symptoms: intrusive thoughts of the deceased, sadness, and yearning for reunion (4-6). Whereas in acute grief these are considered normal, recovery involves a capacity to tolerate reminders of the deceased without symptom induction. Failure to dampen symptoms within 18 months is diagnostic of complicated grief, which develops in 7% to 22% of all mourners and affects roughly 200 million people worldwide each year (5,6). An important clinical question is what neural mechanisms allow mourners to modulate symptoms during the acute phases of grief sufficiently to recover.
We have previously proposed an incentive salience model of grief, in which yearning and intrusive thoughts orient attention toward the unattainable goal of reunion. These must eventually be downregulated; there is debate over whether sadness facilitates or impedes downregulation (7). Three previous imaging studies of bereavement (8-10) have addressed aspects of this model and found support for a role for the nucleus accumbens in mediating yearning in unresolved grief (8). However, none have used behavioral measures of attention to evaluate grief symptomatology. Further, no previous imaging study has evaluated acute grief, occurring within 3 months of the loss, or focused on the likely role of the amygdala in separation distress.
In this study, we sought to extend to bereavement findings that attention indexes threats to living attachments (11), as well as other salient stimuli (12,13). We hypothesized that between-subject differences in mourners' attention toward deceased-related stimuli might identify brain regions mediating intrusive thoughts of the deceased, including the amygdala. We also hypothesized that subjects reporting high levels of intrusive thoughts would demonstrate amygdala hyperactivity on exposure to reminders of their loss, while avoidance would correlate with the opposite pattern.
Hypotheses about attentional and emotional reactivity in grief focused on the amygdala as a candidate neural structure, because several lines of evidence indicate a role for this structure in separation distress. The amygdala is implicated in the subjective experience of sadness (14,15) and more generally in salience encoding and emotional memory (16). It may play a role in detecting social danger (17,18), including threats to attachment (19). Modulatory studies indicate that oxytocin, a neurotransmitter implicated in promoting attachment and bonding, dampens amygdala reactivity to interpersonal threats in humans (20). Juvenile primates with bilateral amygdala lesions, compared with sham and hippocampal control subjects, demonstrate normal attachment behaviors but reduced maternal proximity seeking and distress vocalizations (21). In concert, these findings suggest the previously untested hypothesis that in acutely bereaved human adults, the amygdala may help mediate affective and attentional reactivity to reminders of a deceased attachment, including attentional bias and sadness.
Finally, we examined regulatory processes in acute grief. Consistent with findings of deficits in prefrontal control over amygdala in other affective disorders (22-27), we hypothesized that brain regions that contextualize, monitor, or exert regulatory control over amygdala may modulate grief symptomatology (14,15,28-30). We explored the relationship between the amygdala and three candidate regulatory regions—the dorsolateral prefrontal cortex (DLPFC), the rostral anterior cingulate cortex (rACC), and the dorsal anterior cingulate cortex (dACC). We hypothesized that differences in both attention and grief-related emotion might correlate with the strength of functional connectivity between regulatory regions and the amygdala.
To test these hypotheses, we studied 20 psychiatrically healthy subjects recently bereaved of a pet. Pet bereavement has analogous symptoms to human grief (31), and intensity correlates with attachment strength (32,33). Subjects underwent functional magnetic resonance imaging (fMRI) while completing an Emotional Stroop (ES) task designed to elicit evidence of bias toward reminders of the deceased. Emotional Stroop tasks, pairing emotionally salient words against control words, are an accepted method for examining the relationship between attention and neural activity (13,34). We examined whether attentional bias toward deceased-related words elicited activity in and functional connectivity between salience-processing brain regions. Further, we examined whether two clinically distinct grief styles—intrusiveness and avoidance—correlated with distinct activity patterns.
Immediately following the ES task, subjects visualized being with the deceased, allowing emotion to unfold naturally. The task was not an emotion regulation task, and neural activity was not analyzed. Rather, peak separation distress was measured by questionnaire following mood induction under conditions similar to those seen in the clinical treatment of grief. We hypothesized that relative deficits in functional connectivity between control regions and the amygdala during the ES task might predict vulnerability to grief-related emotion.
Methods and Materials
Participants
The Institutional Review Board of the New York State Psychiatric Institute approved the study and subjects gave informed written consent. Twenty subjects (16 female subjects, 37.8 ± 13.1 years, range = 22–62) who had lost a pet dog or cat (mean ownership 10.6 ± 5 years) within the previous 3 months (mean time since loss 7.3 ± 4.0 weeks; this time period is referred to as acute grief hereafter) were screened for absence of past or current Axis I psychiatric disorders by the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I). Mood over the preceding week was assessed with the Beck Depression Inventory (35) (mean 3.5 ± 3.4, range 0–16). Grief intensity was measured by the Texas Revised Inventory of Grief (TRIG), a 20-item questionnaire in which low scores indicate high grief on a 0 to 84 scale (36) (mean 42.9 ± 14.1, range 0–64). Two cognitive predictors of grief outcome, intrusive and avoidant thoughts, were measured with the Impact of Event Scale-Revised (IES-R) (avoidance 5.8 ± 5.5, range 0 -17; intrusion 10.9 ± 6.6, range 1-22) (37).
Experimental Tasks
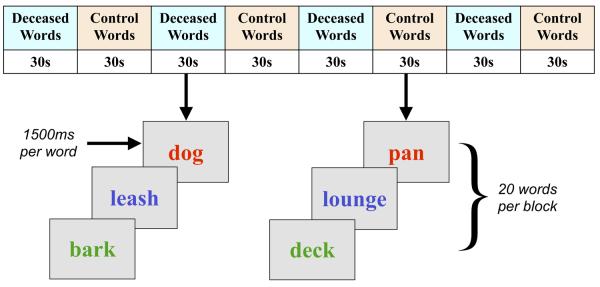
We exposed subjects to two levels of deceased-related attentional load. The low-load condition was an Emotional Stroop task in which subjects named the ink color of previously identified words reminding them either of the deceased or their house, a method allowing control of personal familiarity. Intrinsically emotional words were excluded (e.g., “dog” was allowed but “friend” was not). Sixteen words in each category were matched by length. Blocks of control words alternated with deceased word blocks in an ABABABAB pattern without inter-block intervals, 30 sec per block, following an established paradigm (38) (Figure 1). Order was counterbalanced across subjects. Within each block, 20 randomly selected words were shown for 1500 msec, without fixation, in blue, red, yellow, or green. Subjects were instructed to indicate ink color by button press “as quickly and accurately as possible.” Words remained on the screen for the entire 1500-msec period to ensure equal visual exposure to both word categories. To reduce practice effects, subjects completed four pretask color Stroop blocks. Stimuli were presented using E-Prime software (Psychology Software Tools, Inc., Pittsburgh, Pennsylvania) (39) on a rear projection screen viewed using a mirror suspended from the head coil birdcage.
Figure 1.
Task design. In the Emotional Stroop (ES) task, subjects viewed four blocks of control words alternating with four blocks of deceased-related words. Each word was presented for 1500 msec. Deceased-related stimuli consisted of 16 randomly ordered nonemotional words that reminded subjects of their pet (e.g., “dog” not “friend”); 16 control words reminded subjects of their house (e.g., “bathroom” not “comfortable”). Subjects were told to ignore the semantic content of words and respond as quickly and accurately as possible regarding ink color. Following the ES task, subjects underwent 8 minutes 40 seconds of mood induction, as described in the text. ES, Emotional Stroop task.
For the high-load condition, immediately following the ES task, subjects underwent 8 minutes 40 seconds of deceased memories, five of them living (e.g., “imagine walking with Rover”) and five of them dying (“imagine telling Rover goodbye”). To ensure ecological validity, subjects were instructed to “allow any emotions that occur to arise naturally,” rather than to purposely maximize mood. The original study design called for a control period in which subjects imagined their house rather than their pet, but the first six subjects spontaneously imagined their pet in the house and became emotional; sadness levels were not statistically different from the pet-only subjects (pet = 8.1 ± 2.6/10 versus house = 5.5 ± 3.3/10), and all 20 subjects were included in a between-subjects analysis. As in previous studies of grief (8,10), mood ratings occurred after the task. Subjects were asked to identify emotions experienced during each of the 10 memories and rate peak intensity on a 0 to 10 Likert scale.
Image Acquisition
Images were acquired on a GE 1.5-T scanner (General Electric Medical Systems, Milwaukee, Wisconsin) parallel to the anterior commissure-posterior commissure (AC-PC) line with a T2*-weighted echoplanar imaging (EPI) sequence of 32 contiguous slices (repetition time [TR] = 3000 msec, echo time [TE] = 40 msec, flip angle = 60, field of view [FOV] = 192 × 192 mm, array size 64 × 64) of 3 mm thickness and 3 × 3 in-plane resolution. Structural images were acquired with a T1-weighted spoiled gradient recalled (SPGR) sequence (TR = 19 msec, TE = 6 msec, flip angle = 20, FOV = 256 mm) recording 186 slices at a slice thickness of 1 mm and in-plane resolution of 1 × 1 mm.
Behavioral Data Analysis
SPSS (SPSS, Chicago, Illinois) (35) was used to calculate reaction times (RTs) for correct trials with RTs > 200 msec. A paired t test examined whether RTs were significantly longer for deceased versus control words. Attentional (RT) bias was calculated as (RT for deceased-related words - RT for control words)/(RT for control words) following standard methods (40). A two-tailed Pearson's correlation coefficient was calculated for the correlation of RT bias and intrusiveness and post hoc for RT bias and intrusiveness controlling for TRIG.
Image Data Analysis
Individual fMRI Data
All preprocessing and statistical analyses used FSL 4.0 (http://www.fmrib.ox.ac.uk/fsl) (34). Images were motion corrected (41) (mean relative displacement = .04 ± .3 mm; range .03–.09 mm; mean absolute displacement = .23 ± .1 mm; range .1–.39 mm) and intensity normalized using a mean-based approach (four-dimensional grand mean scaling ensuring comparability between subjects at the group level). Functional data were slice-time corrected and spatially realigned to the first volume. The structural scan was coregistered to the functional images, and transformation parameters were calculated for spatial warping to the Montreal Neurological Institute (MNI) template brain (resampled voxel size: 2 mm3). Normalized functional images were spatially smoothed with a 9-mm3 kernel. The first two volumes (6 seconds) of each run were discarded prior to building and estimating the statistical models. Data were high-pass filtered (Gaussian-weighted least squares straight-line fitting, with sigma = 100 sec). Preprocessed images were entered into a multiple linear regression analysis with a single regressor representing the duration of deceased word blocks, convolved with a canonical double-gamma hemodynamic response function (HRF).
Group fMRI Data
At the whole-brain level, RT bias was used as a covariate in the general linear model (GLM) to explain differences in blood oxygenation level-dependent (BOLD) signal between the deceased and neutral conditions. Results were voxel thresholded at p < .01, cluster size thresholded at p < .01; in a post hoc analysis, common activations to deceased words were voxel thresholded at p < .01, cluster size thresholded at p < .05. Regions of interest (ROIs) used for salience-processing (amygdala) and attentional control regions (DLPFC) were based on results of the whole-brain analysis above, as well dorsal and rostral anterior cingulate cortex (ACC) identified in previous work in our laboratory (22). Masks were adapted from the Harvard-Oxford cortical and subcortical atlases (34). In the first analysis, intrusiveness and avoidance scores, and in the second analysis, the top two reported emotions, sadness and yearning/missing, were entered simultaneously as covariates into the GLM with differences in BOLD as the dependent variable. Region of interest data were resel (small volume) corrected at p < .05 following FSL protocol (42). All peak coordinates reported in the results section are given in xyz format in Talairach and Tournoux space (43). Figures show clusters in MNI-152 space.
Task-Dependent Functional Connectivity
Time series were detrended using a high-pass filter using Gaussian-weighted least squares straight-line fit with sigma = 100 sec. Autocorrelation was controlled with prewhitening using FILM. Time series were intensity normalized using grand mean scaling, as in previous functional connectivity studies using FSL (44,45). Motion correction was applied using MCFLIRT, and nuisance motion parameters were removed using ordinary least squares (OLS) regression. Mean time series were extracted using binarized cluster activation maps from the previous ROI analyses. Multiple methods currently exist for assessing functional connectivity (FC) (46). We employed a novel method that allowed us to examine differences in connectivity between neutral and deceased words. Each time series was segmented into blocks corresponding to the affect and neutral conditions of the experiment. Blocks were then demeaned and affect and neutral blocks concatenated to create separate time series for these two conditions. This allowed us to calculate the functional connectivity during each of the two conditions, as well as the change in connectivity between conditions. To ensure that spikes/shifts did not occur at the transition points between blocks, we measured the difference score between each pair of consecutive time points, calculated the mean difference score and standard deviation, and then determined the number of standard deviations above the mean for the three transition-point pairs. None of the five ROIs demonstrated a change in activity > 1.07 Z scores and therefore spikes were not deemed to be present. Pearson's correlation coefficients were calculated separately for deceased and neutral blocks for the control region-amygdala ROI pairs. Correlation coefficients were converted to normally distributed Z scores, neutral subtracted from deceased blocks, and the difference score was determined. Task-dependent functional connectivity (TDFC) was determined by calculating the Pearson's correlation coefficient for the behavioral measures of interest with the Z score of the FC between the two ROIs.
Results
Attentional Bias Toward the Deceased
Behavioral Results
Subjects demonstrated significant RT bias toward deceased versus control words (deceased mean = 881 ± 142, neutral mean = 832 ± 131; mean difference 48.7 ± 58 msec; 95% confidence interval [CI] = 21.4–75.9; t = 3.8; df = 19; p = .001). Subjects made more errors during the deceased word trials (deceased mean = 14 ± 10, neutral mean = 10.4 ± 7.3; mean difference 3.6 ± 6.6; 95% CI = .45–6.7; t = 2.4; df = 19, p = .027).
Imaging Results
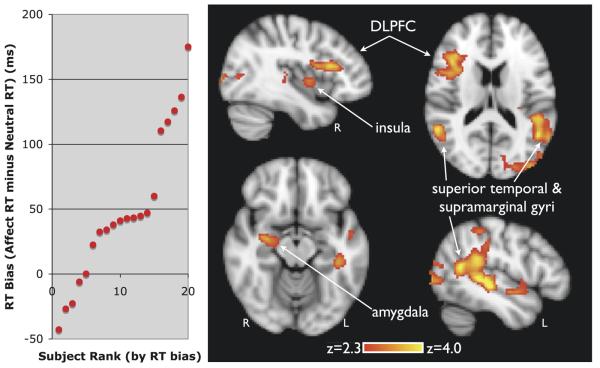
Attentional bias toward deceased words correlated with activity in right amygdala, centered on the ventral portion [xyz = 26 −10 −8; Z = 3.22; p < .01], right insula [xyz = 30 10 8; Z = 3.18; p < .01], right inferior frontal gyrus, referred to as dorsolateral prefrontal cortex (DLPFC) [xyz = 37 17 20; Z = 3.38; xyz = 35 23 20; Z = 3.27; xyz = 49 25 13; Z = 3.24; p <.01], left middle temporal gyrus [xyz = −44 −28 −2; Z = 4.03; p < .01] left superior temporal gyrus [xyz = −46 −36 3; Z = 3.81; p < .01], bilateral angular gyrus [left xyz = −46 −54 14; Z = 3.57; right xyz = 52 −51 13; Z = 3.71; p < .01], left supramarginal gyrus [xyz = −58 −43 28, Z = 4.46 p < .01], and left lateral superior occipital pole [xyz = −39 −85 16; Z = 3.76; xyz = −22 −87 18; Z = 3.73; xyz = −31 −83 22; Z = 3.64; p < .01] (Table 1; Figure 2). No deactivations were noted. Common activations to deceased words are listed in Table S1 in the Supplement 1.
Table 1.
Neural Activity Correlating with Attentional Bias Toward Deceased-Related Words
| T&T Coordinates |
||||
|---|---|---|---|---|
| Regions (activation) | Z Score | x | y | z |
| Supramarginal Gyrus, Posterior (left) | 4.46 | −58 | −43 | 28 |
| Middle Temporal Gyrus, Posterior (left) | 4.03 | −44 | −28 | −2 |
| Superior Temporal Gyrus, Posterior (left) | 3.81 | −46 | −36 | 3 |
| Lateral Occipital Cortex, Superior (left) | 3.76 | −39 | −85 | 16 |
| Lateral Occipital Cortex, Superior (left) | 3.73 | −22 | −87 | 18 |
| Lateral Occipital Cortex, Superior (left) | 3.64 | −31 | −83 | 22 |
| Angular Gyrus (left) | 3.57 | −46 | −54 | 14 |
| Angular Gyrus (right) | 3.71 | 52 | −51 | 13 |
| Inferior Frontal Gyrus (right) | 3.38 | 37 | 17 | 20 |
| Inferior Frontal Gyrus (right) | 3.27 | 35 | 23 | 20 |
| Inferior Frontal Gyrus (right) | 3.24 | 49 | 25 | 13 |
| Amygdala, Ventral (right) | 3.22 | 26 | −10 | −8 |
| Insula (right) | 3.18 | 30 | 10 | 8 |
Whole-brain analysis, voxel p < .01, cluster p <.01, corrected. Local maxima reported.
T&T, Talairach and Tournoux.
Figure 2.
Neural correlates of attentional bias in grief. On the y axis (left panel) the reaction time (RT) bias is plotted. It was calculated as the mean response time (RT) to deceased words minus mean response to control words, excluding error trials. The x axis represents each subject's relative rank by magnitude of bias. Response time bias was used to identify brain regions mediating attention toward the deceased (right panel). Implicated regions included the right insula, right amygdala, left angular gyrus, superior temporal gyrus, supramarginal gyrus, dorsolateral prefrontal cortex (DLPFC), and occipital cortex (voxel threshold p < .01; cluster threshold p < .01). Coordinates and Z scores are shown in Table 1. DLPFC, dorsolateral prefrontal cortex; RT, reaction time.
Attention and Grief Style
Behavioral Results
Intrusiveness (IES-R, intrusiveness) correlated with total grief (TRIG) (r = .863; p < .000; n = 20) (Figure S1 in Supplement 1). Avoidance, intrusiveness, and overall grief severity did not correlate with attentional bias. Intrusiveness controlled for TRIG correlated with attentional bias toward deceased-related words (r = .585; n = 20; p < .007; n = 20) (Figure S1 in Supplement 1).
Imaging Results
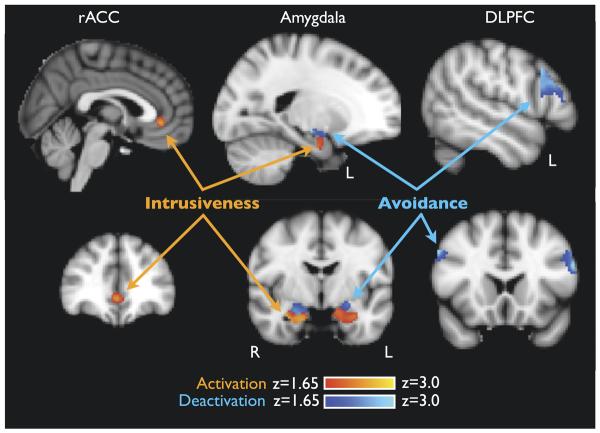
In a double dissociation, avoidance correlated with bilateral dorsal amygdala deactivation to deceased-related words [left xyz = −21 −13 −8; Z = 2.78; right xyz = 17 −1 −14; Z = 2.50; p < .05] while intrusiveness correlated with bilateral ventral amygdala activation [left xyz = −27 −16 −19; Z = 2.43; right xyz = 28 −7 −20; Z = 3.11; p < .05] (Table 2, Figure 3). Avoidance and intrusiveness showed a double dissociation with activity in control regions. Avoidance correlated with deactivation to deceased-related words in bilateral middle frontal gyrus, referred to as DLPFC [left xyz = −52 11 35; Z = 3.75; p < .05; right xyz = 50 12 37; Z = 3.72; p < .05]; intrusiveness correlated with activation in midline rACC extending to both hemispheres but with peak activity on the right [right xyz = 3 34 2; Z = 2.48; p < .05]. No dACC activity was detected.
Table 2.
Activity Correlating with Intrusiveness and Avoidance
| T&T Coordinates |
||||
|---|---|---|---|---|
| Z Score | x | y | z | |
| Avoidance (deactivations) | ||||
| Amygdala, dorsal (left) | 2.78 | −21 | −13 | −8 |
| Amygdala, dorsal (right) | 2.50 | 17 | −1 | −14 |
| Middle frontal gyrus, BA44 (left) | 3.75 | −52 | 11 | 35 |
| Middle frontal gyrus, BA44 (right) | 3.72 | 50 | 12 | 37 |
| Intrusiveness (activations) | ||||
| Amygdala, ventral (left) | 2.43 | −27 | −16 | −19 |
| Amygdala, ventral (right) | 3.11 | 28 | −7 | −20 |
| Cingulate gyrus, rostral anterior (right) | 2.48 | 3 | 34 | 2 |
ROI analyses of DLPFC, rACC, and amygdala (p < .05, corrected). Local maxima reported.
BA, Brodmann area; DLPFC, dorsolateral prefrontal cortex; rACC, rostral anterior cingulate cortex; ROI, region of interest; T&T, Talairach and Tour-noux.
Figure 3.
Neural correlates of cognitive grief style. Avoidance and intrusiveness were entered simultaneously as covariates into the GLM with differences in BOLD as the dependent variable. Regions of interest (ROI) were amygdala (left and right, separately), rostral anterior cingulate cortex (rACC), dorsal ACC (dACC), and dorsolateral prefrontal cortex (DLPFC). Within both the amygdala ROIs, intrusiveness correlated with activation (red) of ventral amygdala, while avoidance correlated with deactivation (blue) of dorsal amygdala. In the control regions, rACC activation correlated with intrusiveness, while DLPFC deactivation correlated with avoidance. Dorsal ACC was not correlated with either measure (p < .05, corrected). Coordinates and Z scores are shown in Table 1. ACC, anterior cingulate cortex; BOLD, blood oxygenation level-dependent; GLM, general linear model.
Mood Induction
Behavioral Results
During mood induction, subjects reported vivid imagery of the deceased, and the strongest emotions induced were sadness (7.3 ± 3.0, range 0–10) and yearning/missing (6.4 ± 3.2, range 0–10). Sadness and yearning were correlated (r = .519; p = .023). Attentional bias did not predict the intensity of any emotion.
Imaging Results
Amygdala activity during the ES task predicted the induced levels of sadness, but not yearning, during the autobiographical memory task (left xyz = −27 −6 −19; Z = 2.03; right xyz = 30 −5 −16; Z = 2.35; p < .05 corrected) (Table 3). No other ROI predicted induced emotion.
Table 3.
Baseline Activity Predicting Sadness During Memory Task
| T&T Coordinates |
||||
|---|---|---|---|---|
| Sadness | Z Score | x | y | z |
| Amygdala (left) | 2.03 | −27 | −6 | −19 |
| Amygdala (right) | 2.35 | 30 | −5 | −16 |
ROI analyses (p < .05, corrected). Local maxima reported.
ROI, region of interest; T&T, Talairach and Tournoux.
Neural Circuitry and Psychological Processes
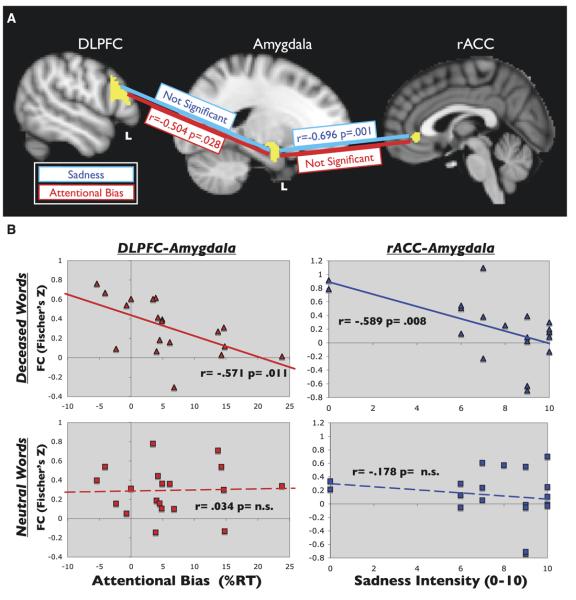
There was a double dissociation between regulatory region and type of task-dependant FC with amygdala. Attentional bias, but not induced emotions, negatively correlated with the change in FC between neutral and affect blocks in left DLPFC-left amygdala (left r = −.504, p = .028; right r = −.300; p = ns) (Figure 4A). Conversely, sadness and yearning/missing, but not attentional bias, correlated with the FC change between rACC-left amygdala (r = −.696 p = .001) and trending towards rACC-right amygdala (r = −.430 p = .066) (Figure 4A). Grief (TRIG, IES-R) and depression (Beck Depression Inventory) scores did not predict FC.
Figure 4.
Task dependent functional connectivity (TDFC) in grief. (A) TDFC was determined by calculating the Pearson's correlation coefficient for attentional and emotional measures of interest with the z score of the change in functional connectivity between neutral and affect blocks. In the ROI pair of left DLPFC-left amygdala, attentional (RT) bias to deceased words, but not emotion, negatively correlated with the change in functional connectivity (FC) (r = −.504, p = .028). In the ROI pair rACC-left amygdala, sadness (and yearning, not shown), but not attention, correlated with the change in FC (r = −.696, p = .001). (B) To examine the basis for the results in (A), TDFC was examined for deceased-word and control-word blocks separately. Subjects with greater levels of attentional control (as indicated by decreased attentional bias) demonstrated greater FC between left DLPFC and left amygdala than those with lower control during the deceased word blocks (r = .571, p = .011) (top left), but not neutral blocks (bottom left). In the rACC-left amygdala pair, FC was lowest in those who subsequently reported high levels of sadness during deceased-word (top right) but not neutral (bottom right) blocks (r = −.589, p = .008). The x axes represent attentional bias as percent increase to deceased versus control words (left) and peak sadness (right). The y axes represent the correlation coefficient for FC between left DLPFC-left amygdala (left) and rACC-left amygdala (right). DLPFC, dorsolateral prefrontal cortex; FC, functional connectivity; rACC, rostral anterior cingulate cortex; ROI, regions of interest; RT, reaction time; TDFC, task dependent functional connectivity.
To explore the basis for the change in connectivity, attention and emotion measures were correlated independently with the FC during deceased blocks and the FC during neutral blocks (Figure 4B). For the ROI pair of DLPFC-amygdala, attentional bias correlated with FC during deceased blocks but not control blocks in the left hemisphere. Specifically, subjects with greater levels of attentional control (less attentional bias) demonstrated greater FC than those with less control (left r = −.571, p = .011; right r = −.429, p = .067) during the deceased word but not neutral blocks. In the rACC-amygdala pair, FC was lowest in those who subsequently reported high levels of sadness and yearning during deceased-word but not neutral blocks (left r = −.589, p = .008; right r = −.441, p = ns).
These findings indicate that subjects with greater attentional control and decreased sadness demonstrated higher functional connectivity between regulatory regions and the amygdala, relative to subjects who had greater grief symptomatology. This appears to drive the change scores reported in Figure 4A. Paired t tests did not reveal a group level FC difference between the neutral and deceased blocks. Descriptive statistics of the FC data are given in Table S2 in Supplement 1.
Discussion
This is the first study to examine the neural correlates of three cardinal symptoms of acute grief (grief occurring within 3 months of a loss). We found that unique patterns of brain activity indexed attention toward reminders of the deceased, grief styles involving distinct cognitive strategies for coping with thoughts of the deceased, and sadness and yearning induced by memories of the deceased.
In the incentive salience model of grief (7), excessive attention toward an unavailable attachment figure is hypothesized to maintain grief's core symptoms. Consistent with this hypothesis, we found that bereaved subjects pay more attention to deceased-related than control words and do so in proportion to ratings of intrusive thoughts. This finding extends to bereavement results from the attachment literature in which nonbereaved subjects demonstrate attentional bias toward subliminal threats of interpersonal loss (47) and follows standard interpretations of the ES task (13,48-51). Of note, because intrinsically emotional words were excluded (e.g., “dog” was permitted but “friend” was not), we interpret attentional bias as reflecting the mourner's current concerns about the deceased (12) and salience valuations of the concept of the deceased (12,13), rather than their evaluation of intrinsic word meaning.
Variation in attentional bias was used to identify brain regions involved in deceased-related processing, including amygdala, insula, and temporoparietal regions (Figure 2). As hypothesized, these are salience encoding and prefrontal regulatory regions. Amygdala activity is consistent with a role in detecting separation from caregivers and symptoms of separation distress (20,21). The insula has direct connections to the amygdala (52), and in other disorders of salience processing, it correlates with attentional bias toward reminders of the unattainable reward (53). The temporal and parietal regions activated here contribute to representations of self and others (54-56) but a more conservative interpretation emphasizes their role in detecting word salience (57-61). This finding may help to explain the clinical experience of grief, in which reminders of the deceased are experienced as particularly meaningful (62).
Next, we examined whether variation in two cognitive symptoms of grief, intrusiveness and avoidance, correlated with differential activity in amygdala and prefrontal regulatory regions. These two grief styles are clinically distinct, with avoidant patients rarely presenting for therapy, while those with intrusive experiences often requiring treatment and often going on to develop complicated grief (4-6). We expected that the more emotionally distressing style, intrusiveness, would correlate with amygdalar hyperactivity and control region hypoactivity.
Amygdalar results were consistent with the hypothesis. In a single ROI, we found a double dissociation between dorsal and ventral subregions and grief style (Figure 3). Avoidance correlated with dorsal amygdala dampening while intrusiveness correlated with ventral amygdala activation. Results are consistent with a model in which the amygdala is sensitive to reminders of loss, while intrusiveness and avoidance respectively represent “top down” and “bottom up” processes that modulate this amygdala reactivity. This is consistent with previous work in which ventral amygdala activity correlates with exposure to unconscious (bottom up) stimuli and dorsal amygdala activations correlate to conscious (top down) stimuli (24).
Regulatory region and grief style demonstrated a double dissociation (Figure 3), inverse to the hypothesized pattern. The finding that rACC activation covaries with amygdala activity is consistent with a role for this region in monitoring and contextualizing salience detection activity in amygdala (14,15,28-30). Future research should seek to explain the finding of DLPFC deactivation covarying with avoidance by examining whether stimulus dampening at an earlier stage of processing, downregulation of salience earlier in bereavement, or decreased baseline attachment cause dampened DLPFC activity.
The third major grief symptom examined was emotional responses to memories of the deceased. Although epidemiological data indicate yearning is the primary emotional symptom in grief, clinical data emphasize sadness as the most common peak emotion (63). Consistent with this, sadness was the strongest emotion elicited by memories of the deceased, followed by yearning/missing.
Consistent with the hypothesis that amygdala is implicated in sad emotion related to attachment loss (14,15), we found that bilateral amygdalar activity, but not rACC or DLPFC activity, predicted peak levels of subsequent sadness (Table 3).
We found support for a model in which the rACC and other medial prefrontal structures monitor (29) and contextualize (2) information processing in the amygdala, a process that may affect the expression of grief related emotion. Figure 4B depicts a trend from high functional connectivity toward zero FC between rACC and amaygala, as sadness intensity increased. Low connectivity scores between rACC and amygdala may indicate low levels of contextualization, thereby releasing amygdala reactivity, while higher levels of contextualization may indicate improved emotion regulation capacity during mood induction. This hypothesis must be tested in designs in which rACC-amygdala functional connectivity during low-load exposure is correlated with functional connectivity between control regions and the amygdala during explicit efforts to reduce sadness during mood provocation.
We also found evidence supporting a role for the DLPFC in maintaining attentional set on tasks unrelated to grief in the presence of grief-related distractors, a problem seen during clinical grief. DLPFC-amygdala functional connectivity negatively covaried with attentional bias towards deceased words (26). This is consistent with recent functional connectivity evidence from an fMRI time-series analysis that shows that DLPFC activity is coupled with amygdala activity during cognitive tasks in normal, relative to depressed, subjects (64).
The study had several limitations. Its aim was to understand variation in grief responses and therefore employed a between-subjects and not a between-group design, as in several previous studies of grief (10,65,66). The small number of male subjects makes gender comparisons unreliable. Future studies with larger sample sizes may permit exploration of the important question of male and female subject differences. The finding that differences in rACC-amygdala FC predicted subsequent grief-related emotion is not itself evidence of regulatory deficiencies during the emotion but can be used to guide future hypotheses.
Conclusions
This study found evidence consistent with the previously untested hypothesis that the amygdala, a brain region implicated in separation distress in primates (21) may contribute to separation distress reactions following exposure to reminders of a deceased attachment during acute human bereavement. Further, consistent with findings of deficits in prefrontal control over amygdala in affective disorders (22-27), we found that variation in prefrontal activity and functional connectivity with amygdala predicted variation in grief-related symptoms. These results support a model in which the amygdala mediates core attentional and emotional symptoms of separation distress, including sadness, while two prefrontal regions, DLPFC and rACC, modulate attentional and emotional aspects of amygdala reactivity, respectively. Findings have implications for the study and treatment of grief-related psychopathology.
Supplementary Material
Acknowledgments
This study was supported by grants from the Hope for Depression Research Foundation (HDRF) and the Neuropsychoanalysis Foundation. The granting agencies had no role in any of the following aspects of this study: design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. PJF was supported by a National Institute of Mental Health T32 Training Grant in Anxiety and Related Disorders. TKY was supported by a 5T32 National Institutes of Health (NIH) Medical Scientist Training Program Training Grant. Authors PJF, TKY, JH and JJM are independent of any commercial provider, had full access to all of the data in this study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
We thank Jack Grinband for his incisive help with analysis of design and results; Todd Ogden for his assistance with statistical analysis; Victoria Arango for neuroanatomical training; Amit Etkin and Tobias Enger for assistance on theoretical framework; and Kevin Desimone for assistance in functional connectivity analysis.
Footnotes
The authors reported no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Rosenblatt P, Walsh R, Jackson D. Grief and Mourning in Cross-Cultural Perspective. Human Relations Area File Press; New Haven, CT: 1976. [Google Scholar]
- 2.Kagawa-Singer M. The cultural context of death rituals and mourning practices. Oncol Nurs Forum. 1998;25:1752–1756. [PubMed] [Google Scholar]
- 3.Archer J. Grief from an evolutionary perspective. In: Stroeb M, Hansson R, Stroeb W, Schut H, editors. Handbook of Bereavement Research: Consequences, Coping, and Care. American Psychological Association; Washington, DC: 2001. [Google Scholar]
- 4.Maciejewski PK, Zhang B, Block SD, Prigerson HG. An empirical examination of the stage theory of grief. JAMA. 2007;297:716–723. doi: 10.1001/jama.297.7.716. [DOI] [PubMed] [Google Scholar]
- 5.Lichtenthal W, Cruess D, Prigerson H. A case for establishing complicated grief as a distinct mental disorder in DSM-V. Clin Psychol Rev. 2004;24:637–662. doi: 10.1016/j.cpr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Forstmeier S, Maercker A. Comparison of two diagnostic systems for complicated grief. J Affect Disord. 2007;99:203–211. doi: 10.1016/j.jad.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Freed PJ, Mann JJ. Sadness and loss: Toward a neurobiopsycho-social model. Am J Psychiatry. 2007;164:28–34. doi: 10.1176/ajp.2007.164.1.28. [DOI] [PubMed] [Google Scholar]
- 8.O'Connor MF, Wellisch DK, Stanton AL, Eisenberger NI, Irwin MR, Lieberman MD. Craving love? Enduring grief activates brain's reward center. Neuroimage. 2008;42:969–972. doi: 10.1016/j.neuroimage.2008.04.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Connor MF, Gündel H, McRae K, Lane RD. Baseline vagal tone predicts BOLD response during elicitation of grief. Neuropsychopharmacology. 2007;32:2184–2189. doi: 10.1038/sj.npp.1301342. [DOI] [PubMed] [Google Scholar]
- 10.Gundel H, O'Connor M, Littrell L, Fort C, Lane R. Functional neuroanatomy of grief: An fMRI study. Am J Psychiatry. 2003;160:1946–1953. doi: 10.1176/appi.ajp.160.11.1946. [DOI] [PubMed] [Google Scholar]
- 11.Mikulincer M, Doleve T, Shaver P. Attachment-related strategies during thought-suppression: Ironic rebounds and vulnerable self-representations. J Pers Soc Psychol. 2004;87:940–956. doi: 10.1037/0022-3514.87.6.940. [DOI] [PubMed] [Google Scholar]
- 12.Cox WM, Fadardi JS, Pothos EM. The addiction-stroop test: Theoretical considerations and procedural recommendations. Psychol Bull. 2006;132:443–476. doi: 10.1037/0033-2909.132.3.443. [DOI] [PubMed] [Google Scholar]
- 13.Franken I. Drug craving and addiction: Integrating psychological and neuropsychopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, McCarthy G, Song AW, Labar KS. Amygdala activation to sad pictures during high-field (4 tesla) functional magnetic resonance imaging. Emotion. 2005;5:12–22. doi: 10.1037/1528-3542.5.1.12. [DOI] [PubMed] [Google Scholar]
- 15.Goldin PR, Hutcherson CA, Ochsner KN, Glover GH, Gabrieli JD, Gross JJ. The neural bases of amusement and sadness: A comparison of block contrast and subject-specific emotion intensity regression approaches. Neuroimage. 2005;27:26–36. doi: 10.1016/j.neuroimage.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 17.Amaral DG. The primate amygdala and the neurobiology of social behavior: Implicaitons for understanding social anxiety. Biol Psychiatry. 2002;51:11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- 18.Panksepp J. Affective Neuroscience. Oxford University Press; New York: 1998. Loneliness and the social bond: The brain sources of sorrow and grief; pp. 261–279. [Google Scholar]
- 19.Lemche E, Giampietro VP, Surguladze SA, Amaro EJ, Andrew CM, Williams SC, et al. Human attachment security is mediated by the amygdala: Evidence from combined fMRI and psychophysiological measures. Hum Brain Mapp. 2006;27:623–635. doi: 10.1002/hbm.20206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. J Neurosci. 2004;24:711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 23.Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacDonald A, Cohen J, Stenger V, Carter C. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 27.Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63:377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 30.Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: A critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- 31.Archer J, Winchester G. Bereavement following death of a pet. Br J Psychol. 1994;85:259–271. doi: 10.1111/j.2044-8295.1994.tb02522.x. [DOI] [PubMed] [Google Scholar]
- 32.Adams CL, Bonnett BN, Meek AH. Predictors of owner response to companion animal death in 177 clients from 14 practices in Ontario. J Am Vet Med Assoc. 2000;217:1303–1309. doi: 10.2460/javma.2000.217.1303. [DOI] [PubMed] [Google Scholar]
- 33.Wrobel T, Dye A. Grieving pet death: Normative, gender, and attachment issues. Omega: The Journal of Death and Dying. 2003;47:385–393. [Google Scholar]
- 34.Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, et al. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144:1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beck AT, Ward C, Mendelson M. Beck Depression Inventory (BDI) Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 36.Faschingbauer T, Zisook S, Devaul R. The Texas Revised Inventory of Grief. In: Zisook S, editor. Biopsychosocial Aspects of Bereavement. American Psychiatric Press; Washington, DC: 1987. pp. 111–124. [Google Scholar]
- 37.Weiss DS, Marmar C. The Impact of Event Scale-Revised. In: Wilson J, Keane T, editors. Assessing Psychological Trauma and PTSD. Guildford; New York: 1997. [Google Scholar]
- 38.Whalen P, Bush G, McNally R, McInerney S, Jenike MA, Rauch SL. The emotional counting Stroop paradigm: A functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- 39.Schneider W, Eschman A, Zuccolotto A. E-Prime User's Guide. Psychology Software Tools, Inc; Pittsburgh: 2002. [Google Scholar]
- 40.Algom D, Chajut E, Lev S. A rational look at the emotional Stroop phenomenon: A generic slowdown, not a Stroop effect. J Exp Psychol Gen. 2004;133:323–338. doi: 10.1037/0096-3445.133.3.323. [DOI] [PubMed] [Google Scholar]
- 41.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 42.Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 43.Fox PT, Laird AR, Fox SP, Fox PM, Uecker AM, Crank M, et al. BrainMap taxonomy of experimental design: Description and evaluation. Hum Brain Mapp. 2005;25:185–198. doi: 10.1002/hbm.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- 45.Sorg C, Riedl V, Mühlau M, Calhoun VD, Eichele T, Läer L, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers BP, Morgan VL, Newton AT, Gore JC. Assessing functional connectivity in the human brain by fMRI. Magn Reson Imaging. 2007;25:1347–1357. doi: 10.1016/j.mri.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mikulincer M, Gillath O, Shaver P. Activation of the attachment system in adulthood: Threat-related primes increase the accessibility of mental representations of attachment figures. J Pers Soc Psychol. 2002;83:881–895. [PubMed] [Google Scholar]
- 48.Dalgleish T, Power M, editors. Handbook of Cognition and Emotion. John Wiley & Sons; New York: 1999. [Google Scholar]
- 49.Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- 50.Compton R, Banich M, Mohanty A, Milham M, Herrington J, Miller G, et al. Paying attention to emotion: An fMRI investigation of cognitive and emotional Stroop tasks. Cogn Affect Behav Neurosci. 2003;3:81–96. doi: 10.3758/cabn.3.2.81. [DOI] [PubMed] [Google Scholar]
- 51.Robbins SJ, Ehrman RN. The role of attentional bias in substance abuse. Behav Cogn Neurosci Rev. 2004;3:243–260. doi: 10.1177/1534582305275423. [DOI] [PubMed] [Google Scholar]
- 52.Price JL. Comparative aspects of amygdala connectivity. Ann N Y Acad Sci. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- 53.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Platek SM, Keenan JP, Gallup GG, Mohamed FB. Where am I? The neurological correlates of self and other. Brain Res Cogn Brain Res. 2004;19:114–122. doi: 10.1016/j.cogbrainres.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 55.Leslie KR, Johnson-Frey SH, Grafton ST. Functional imaging of face and hand imitation: Towards a motor theory of empathy. Neuroimage. 2004;21:601–607. doi: 10.1016/j.neuroimage.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 56.Buchheim A, Erk S, George C, Kachele H, Ruchsow M, Spitzer M, et al. Measuring attachment representation in an fMRI environment: A pilot study. Psychopathology. 2006;39:144–152. doi: 10.1159/000091800. [DOI] [PubMed] [Google Scholar]
- 57.Obleser J, Wise RJ, Alex Dresner M, Scott SK. Functional integration across brain regions improves speech perception under adverse listening conditions. J Neurosci. 2007;27:2283–2289. doi: 10.1523/JNEUROSCI.4663-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Humphries C, Binder JR, Medler DA, Liebenthal E. Time course of semantic processes during sentence comprehension: An fMRI study. Neuroimage. 2007;36:924–932. doi: 10.1016/j.neuroimage.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raettig T, Kotz SA. Auditory processing of different types of pseudo-words: An event-related fMRI study. Neuroimage. 2008;39:1420–1428. doi: 10.1016/j.neuroimage.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 60.Kuchinke L, Jacobs AM, Grubich C, Võ ML, Conrad M, Herrmann M. Incidental effects of emotional valence in single word processing: An fMRI study. Neuroimage. 2005;28:1022–1032. doi: 10.1016/j.neuroimage.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 61.Gennari SP, MacDonald MC, Postle BR, Seidenberg MS. Context-dependent interpretation of words: Evidence for interactive neural processes. Neuroimage. 2007;35:1278–1286. doi: 10.1016/j.neuroimage.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parkes CM. Bereavement: Studies of grief in adult life. Penguin Books; London: 1998. [Google Scholar]
- 63.Bonanno GA, Goorin L, Coifman K. Sadness and grief. In: Lewis M, Haviland-Jones JM, Barrett L, editors. Handbook of Emotions. 3rd ed. Guilford Press; New York: 2008. pp. 797–810. [Google Scholar]
- 64.Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 65.Najib A, Lorberbaum JP, Kose S, Bohning DE, George MS. Regional brain activity in women grieving a romantic relationship breakup. Am J Psychiatry. 2004;161:2245–2256. doi: 10.1176/appi.ajp.161.12.2245. [DOI] [PubMed] [Google Scholar]
- 66.O'Connor MF, Gündel H, McRae K, Lane RD. Baseline vagal tone predicts BOLD response during elicitation of grief. Neuropsychopharmacology. 2007;32:2184–2189. doi: 10.1038/sj.npp.1301342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.