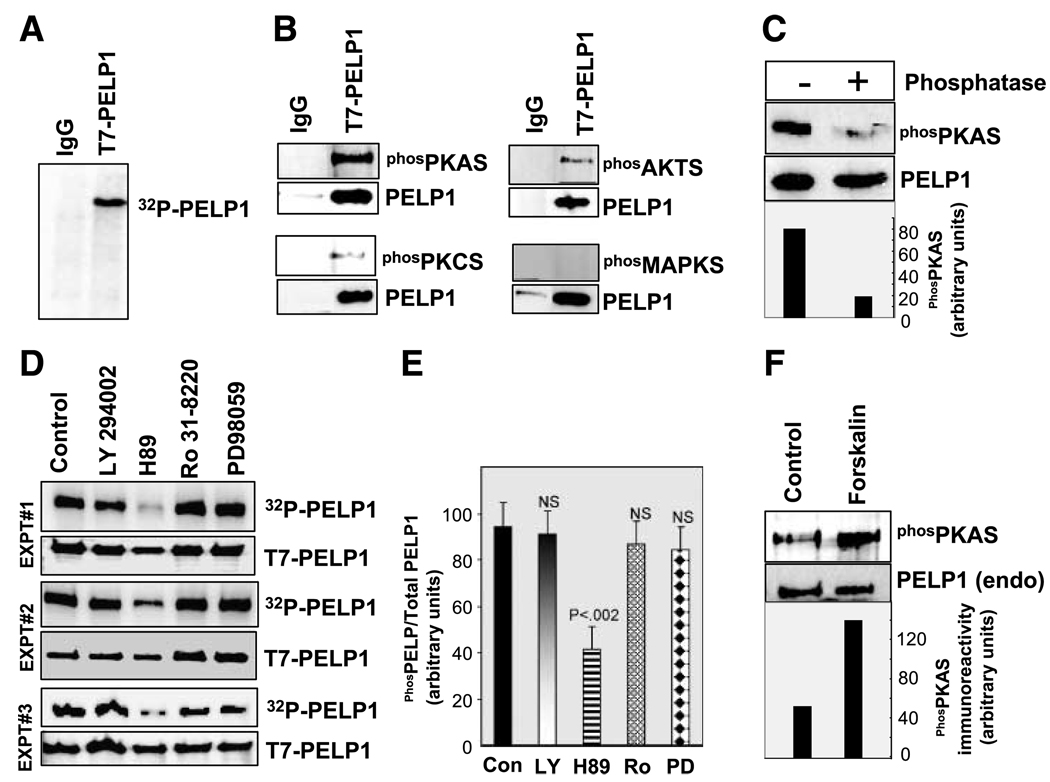
Figure 1.
PELP1 is phosphorylated by serine/threonine kinases in vivo. A. MCF-7-PELP1 cells were labeled with [32P]PI in vivo, and PELP1 phosphorylation status was analyzed by immunoprecipitation and autoradiography. B. PELP1 was immunoprecipitated using a control or the T7-epitope – tagged antibody from total lysates prepared from MCF-7-PELP1 clones and Western blotted with phosphorylated (serine/threonine) substrate – specific antibodies that uniquely recognize PKA, PKC, AKT, or MAPK. C. T7-PELP1 was immunoprecipitated from MCF-7-PELP1 cells and treated with phosphatase, and phosphorylation status was analyzed by using the phosphorylated PKA substrate antibody. D. MCF-7-PELP1 cells were cultured in 10% serum, in vivo labeled with [32P]PI, and treated for 1 h with various inhibitors: LY294002 (phosphatidylinositol-3-kinase inhibitor), H89 (PKA inhibitor), Ro 31-8220 (PKC inhibitor), or PD98059 (MAPK pathway inhibitor). T7-PELP1 was immunoprecipitated, and phosphorylation status was measured by autoradiography. These in vivo experiments are repeated thrice and are shown as experiments 1, 2, and 3. E. Intensity of the bands in D were quantified by densitometry and are shown as bar graph. Columns, average of three experiments and the differences in the intensity were analyzed by t test. P, significance level; NS, not significant. F. MCF-7 cells were cultured in serum-free medium and stimulated with PKA activator forskolin, and the phosphorylation status of endogenous PELP1 was analyzed by immunoprecipitation and autoradiography.