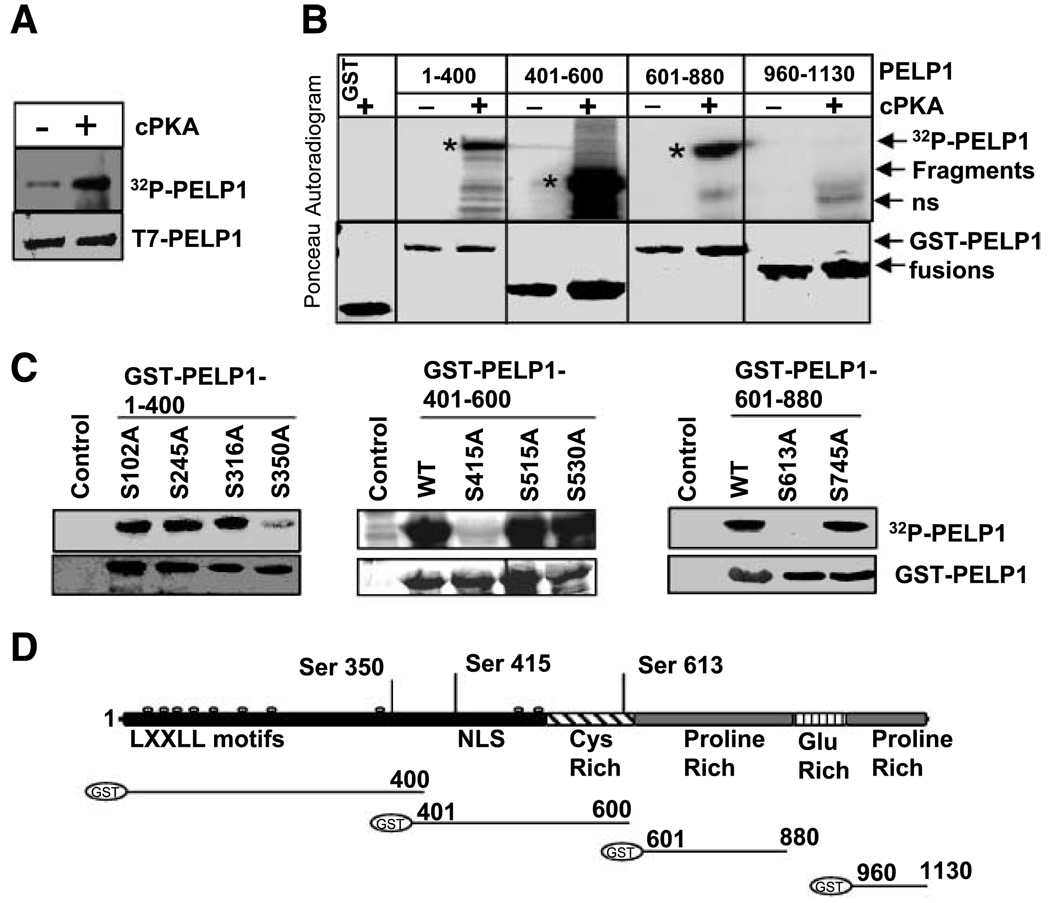
Figure 3.
PELP1 is a substrate of PKA. A. T7-PELP1 was immunoprecipitated from serum-starved MCF-7-PELP1 cells and subjected to in vitro kinase assays using the purified catalytic subunit of PKA (cPKA). B. PELP1-GST fusions of indicated length were subjected to in vitro kinase reaction with [32P]γ-ATP in the presence or absence of catalytic subunit of PKA. *, specific PELP1 GST proteins that are phosphorylated by PKA; ns, nonspecific band. C. In vitro kinase assay of GST-PELP1 mutants that lack specific PKA phosphorylation sites. Mutations were created by using site-directed mutagenesis with GST-PELP1 1–400, 401–600, 601–880 and 960–1130 plasmids as backbones. D. Schematic representation of GST-PELP1 fusions of various PELP1 domains used in this study. Putative PKA phosphorylation sites identified in PELP1 (top).