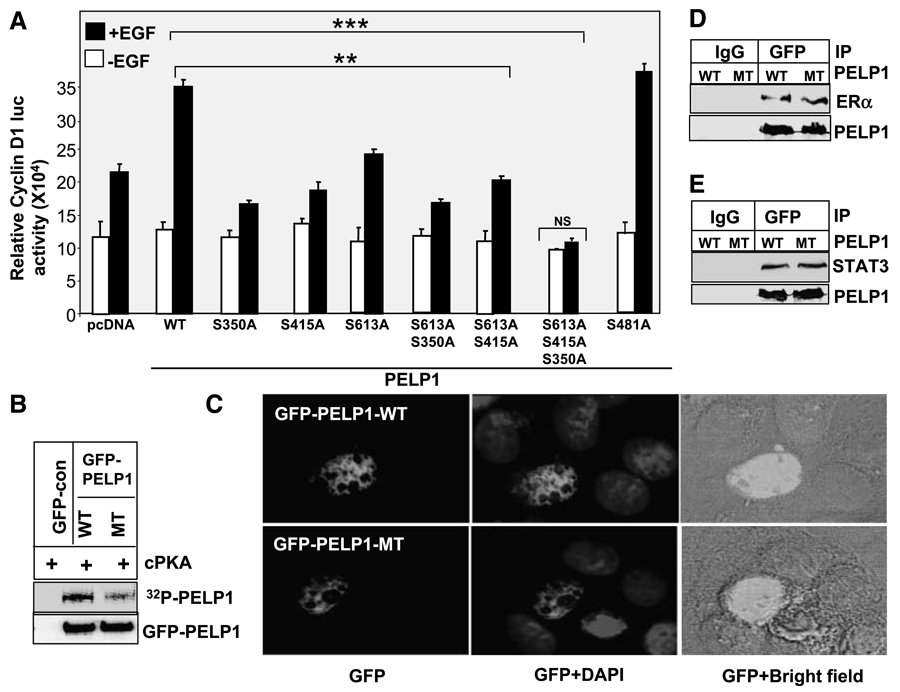
Figure 4.
Growth factor – mediated PELP1 coactivation functions require functional PKA phosphorylation sites. A. Cos1 cells were cotransfected with cyclin D1 – luciferase reporter along with the cDNAs for the pcDNA vector, pcDNA-PELP1-WT, or the PELP1 mutants that lack specific PKA phosphorylation sites. Cells were serum starved for 24 h and stimulated with 100 ng/mL of EGF for 12 h, and the luciferase activity was measured. *, P < 0.05; **, P < 0.001. Columns, mean from three independent experiments done in triplicate wells; bars, SE. B. GFP-PELP1 or GFP-PELP1-MT was transfected into Cos1 cells, and 72 h later, GFP-PELP1-WT and GFP-PELP1-MT were immunoprecipitated from the serum-starved Cos1 cells and subjected to in vitro kinase assays using catalytic subunit of PKA. C. MCF-7 cells were transiently transfected with GFP-tagged PELP1-WT or GFP-tagged PELP1-MT (S350, S415, S613A), and the cellular localization of GFP-PELP1 was determined by using confocal microscopy. D and E. Cos1 were cotransfected with PELP1-WT or PELP1-MT (S350, S415, S613A) plasmids along with ER or STAT3 expression plasmids. After 48 h, cells were stimulated with EGF (100 ng/mL) and PELP1 was immunoprecipitated. The association of the ER and STAT3 with PELP1 was analyzed by Western blot analysis.