Summary
The Janus family kinases (Jaks), Jak1, Jak2, Jak3, and Tyk2, form one subgroup of the non-receptor protein tyrosine kinases. They are involved in cell growth, survival, development, and differentiation of a variety of cells but are critically important for immune cells and hematopoietic cells. Data from experimental mice and clinical observations have unraveled multiple signaling events mediated by Jak in innate and adaptive immunity. Deficiency of Jak3 or Tyk2 results in defined clinical disorders, which are also evident in mouse models. A striking phenotype associated with inactivating Jak3 mutations is severe combined immunodeficiency syndrome, whereas mutation of Tyk2 results in another primary immunodeficiency termed autosomal recessive hyperimmunoglobulin E syndrome. In contrast, complete deletion of Jak1 or Jak2 in the mouse are not compatible with life and, unsurprisingly, do not have counterparts in human disease. However, activating mutations of each of the Jaks are found in association with malignant transformation, the most common being gain-of-function mutations of Jak2 in polycythemia vera and other myeloproliferative disorders. Our existing knowledge on Jak signaling pathways and fundamental work on their biochemical structure and intracellular interactions allow us to develop new strategies for controlling autoimmune diseases or malignancies by developing selective Jak inhibitors, which are now coming into clinical use. Despite the fact that Jaks were discovered only a little more than a decade ago, at the time of writing there are 20 clinical trials underway testing the safety and efficacy of Jak inhibitors.
Keywords: cytokines, immunoregulation, immunosuppression, Janus kinases, leukemia, signal transduction
Introduction
The human genome documented the existence of 518 protein kinases and 90 members of the kinase family that form the major group of protein tyrosine kinases (PTKs) (1). PTKs are enzymes responsible for the transfer of the γ phosphate of a purine nucleotide tripohosphate, such as adenosine triphosphate (ATP) or guanosine triphosphate (GTP), to hydroxyl groups of specific tyrosine residues of their protein substrates. In contrast to the serine/threonine kinases, the substrates of PTKs are tyrosine residues; although, so-called dual kinases can catalyze phosphorylation of both serine/threonine and tyrosine residues. Tyrosine phosphorylation activates enzymatic activity but also allows the recruitment of downstream signaling proteins by changing the conformation and creating binding sites for proteins with Src homology 2 (SH2) domains. In principle, PTK can be divided into two classes, depending on their cellular localization. Receptor tyrosine kinases are transmembrane receptors that have intrinsic kinase domains within the intracellular portions of the receptor. Ligand binding induces receptor dimerization, and autophosphorylation in trans or in cis results in kinase activation and initiates downstream signaling. Epidermal growth factor receptor (EGFR), fms-like tyrosine kinase-3 (FLT-3), or KIT are typical members of the 58 existing receptor PTKs, which are divided into 16 subgroups. In contrast, the cytoplasmic, non-receptor PTK subfamily is composed of 9 subgroups with 32 members. The non-receptor PTKs also transmit signals from extracellular stimuli. After binding to their specific ligand, the stimulated receptors activate associated cytoplasmic PTK, and tyrosine phosphorylation subsequently recruits additional signaling proteins by providing binding sites. The four members of the Janus family kinases (Jaks), Jak1, Jak2, Jak3, and Tyk2, form one subgroup of the non-receptor PTK. Whereas Jak1, Jak2, and Tyk2 are expressed ubiquitously in mammals, Jak3 is primarily expressed in hematopoietic cells (2, 3). Since hematopoietic cytokines and growth factors use the members of the Jak family for signal transduction, Jaks are critically involved in cell growth, survival, development, and differentiation of immune cells. Effective innate and adaptive immune responses require functional Jak signaling to protect the organism from infections or tumors and mutations leading to loss of function make up some of the commonest inherited severe immunodeficiencies. Conversely, activating mutations or mutations leading to functional loss of Jak members cause malignant transformation of lymphocytes or myeloid cells. We now know that a major but not exclusive means by which Jaks exert their effect is through the activation of a relatively small number of latent, cytosolic DNA-binding proteins term the STATs (signal transducers and activators of transcription). Given the importance of what has come to be known as the Jak-STAT pathway, this field has been the subject of numerous, comprehensive reviews (4–8). In this review, we discuss the functional role of Jak-mediated signaling pathways in immune cell differentiation and associated immune diseases, focusing on the many advances that have occurred in the last few years.
Jak protein structure and regulatory mechanisms
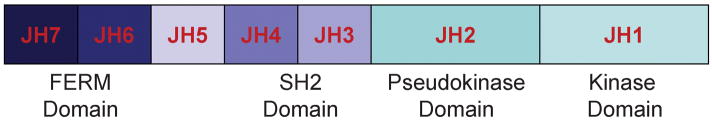
The genes of the four Jak family members in mammals are located on three different chromosomes. The first Jak family member originally identified as a novel class of PTK in human, Tyk2, is located on chromosome 19p13.2 clustered together with the Jak3 gene at 19p13.1 (9, 10). The genes coding for Jak1 and Jak2 are located at chromosome 1p31.3 and 9p24 (11). In mice, the Jak1 gene is located on chromosome 4, Jak2 on chromosome 19, and Jak3 and Tyk2 on chromosome 8. Jaks are relatively large proteins containing more than 1,000 amino acids. Seven distinct Jak homology regions (JH) have been identified (JH1 to JH7), and these form the putative structural domains of the Jak family members (Fig. 1). The catalytically active kinase domain (JH1) is located at the carboxyl-terminus, and at its amino-terminal site, it is directly followed by the enzymatically inactive pseudokinase domain, a unique feature of Jaks among PTKs. Despite the lack of intrinsic kinase activity, the JH2 pseudokinase provides critical regulatory functions. Artificial and disease-associated mutations in the JH2 domain have been shown to positively and negatively regulate Jak kinase activity (12–14). Importantly, a single point mutation (Val617Phe or V617F) within the JH2 pseudokinase domain of Jak2 has been shown to be present in almost all patients with polycythemia vera (PV), as well as high percentages also in patients with essential thrombocythemia (ET), and idiopathic myelofibrosis (15–18). These disorders vividly illustrate the regulatory function of the JH2 domain in human Jak signaling (17). Genetic analysis of families with cases of myeloproliferative disorders (MPD) suggests that the V617F JAK2 mutation is acquired (19). The amino-terminus of Jak contains a SH2-like domain (JH3-JH4) and a 4.1, ezrin, radixin, moesin (FERM) homology domain (JH6 and JH7) (Fig. 1). Mutations within the FERM domain of Jak3 have been reported to impair kinase activity (20). In contrast with its role on the kinase activity, the FERM domain seems to be essential for binding of Jak to its receptor. Chimeric constructs containing the amino-terminal domain of Jak3 conferred to Jak3 or mutated Jak3 proteins containing only the FERM domains JH6 and JH7 but not the other Jak3 domains effectively bind the common γ (γc) chain (21). Similarly, mutation of the hydrophobic residues of the FERM domain led to structural changes, inhibited Jak1 binding to the interferon-γ (IFN-γ) receptor, and impaired inhibition of Jak phosphorylation (22). Mutation of a tyrosine residue within the FERM domain (Y613) of Jak2 results in constitutive Jak2 activation, even in the absence of erythropoietin (Epo) stimulation and even though the site is not phosphorylated (23). Thus, the FERM domain mediates interactions of Jak with its cognate transmembrane cytokine receptor proteins and regulates kinase activity. The role of the SH2 domain (JH3 and JH4) in Jak is not yet clarified, although in other kinases this domain has a key role. The generation of a mutated SH2 domain of Jak1 did not affect its kinase activity and did not interfere with its receptor association (24). The internal regulation of Jak protein kinase activity by the pseudokinase domain and the FERM unit can be further altered during cytokine stimulation.
Fig. 1. Schematic structure of Jaks.
The Jak family comprises four structurally related kinases: Jak1, Jak2, Jak3, and Tyk2. Seven Jak homology regions (JH) containing the catalytically active kinase domain (JH1), the enzymatically inactive pseudokinase domain (JH2), the SH2 domain (JH3, JH4), and a FERM domain (JH6, JH7) form the Jak protein. The FERM domain mediates Jak binding to the transmembrane cytokine receptor and regulates kinase activity.
Regulation of Jaks by phosphorylation
Like other tyrosine kinases, Jak family members are also regulated by protein phosphorylation. For instance, growth hormone (GH) stimulation results in autophosphorylation of Y813 of Jak2, which enables the binding of the SH2 domain- containing adaptor protein SH2-Bβ. Similarly, SH2-Bβ binding to Jak3 requires autophosphorylation of Y785, which is induced by interleukin-2 (IL-2) stimulation (25). For most kinases, autophosphorylation within the activation loop is a critical aspect of positive regulation; although the catalytic activity of Jak3 has been reported to be positively or negatively regulated by tyrosine phosphorylation of tyrosine residues in the activation loop of Jak3 (26). Recently, two new tyrosine phosphorylation sites within the Jak3 protein, Y904 and Y939, were identified, that positively regulate Jak3 activity (27). These tyrosine residues are located within the JH1 kinase domain and undergo phosphorylation after stimulation by cytokines that signal through the Jak3 associated γc chain receptors like IL-2, and also promote kinase activity. The tyrosine residue Y913 of Jak2 is an example of negative regulation of Jak activity. Epo signaling induces phosphorylation of Y913, which is located within the JH1 domain of Jak2, and limits kinase activity of Jak2 and downstream STAT5 activation (28). Autophosphorylation of a tyrosine residue within the FERM domain (Y119) initiates Jak2 dissociation from the Epo receptor and subsequent kinase degradation (29). These examples demonstrate the opposing roles of distinct tyrosine residues within the Jak family members, and the complexity of regulation of Jak kinase activity. Moreover, Jak phosphorylation is regulated by tyrosine phosphatases such as CD45 and SHP-1 (30–32). In contrast, little is known regarding the role of serine phosphorylation sites of Jak. A few reports suggest that Jak kinase activity can also be modified by phosphorylation of serine residues, as shown by S523 phosphorylation of Jak2, inhibiting its kinase activity (33, 34).
A further regulatory mechanism of Jak signaling is mediated by a family of eight intracellular proteins with mainly negative feedback regulation of cytokine signal transduction. These include the suppressor of cytokine signaling (SOCS) proteins and cytokine inducible SH2-domain-containing proteins (CIS). SOCS1 to SOCS7 and CIS all are composed of a central SH2 domain, a carboxy-terminal SOCS box, and an amino-terminal variable domain. SOCS-box containing proteins seem to function as E3 ubiquitin ligases. The amino-terminal region seems to be responsible for protein degradation. Two SOCS proteins, SOCS1 and SOCS3 contain an additional domain inhibiting Jak activity by interaction with their kinase inhibitory region (KIR) that may act as pseudosubstrate (35, 36). Thus, several regulatory mechanisms integrated within the Jak protein or provided by interaction with other proteins exist to control Jak activation.
Functional consequences of cytokine signaling through Jaks
Type I and type II cytokine receptors depend on signal transmission by cytoplasmic, non-receptor PTK like Jak, since they do not have receptor-bound kinase activity. A single Jak or combinations of Jak members selectively associate with different cytokine receptors for transmitting their signals after specific receptor activation. Type II cytokine receptors like receptors for IL-10, IL-19, IL-20, and IL-22 as well gp130 subunit sharing receptors for IL-6 and IL-11 mainly signal through Jak1 but also associate with Jak2 and Tyk2. Receptors for hormone-like cytokines such as GH, prolactin (Prl), Epo, thrombopoietin (Tpo), and cytokine receptors involved in hematopoietic cell development, such as IL-3 or granulocyte-macrophage colony-stimulating factor (GM-CSF) use Jak2 (37, 38). Tyk2 associates with cytokine receptors that signal through various combinations with Jak1 and Jak2 but not with Jak3. The type I IFNs use a combination of Jak1 and Tyk2, and the p40-containing cytokines IL-12 and IL-23 mediate their signals via the combination of Jak2 and Tyk2 (39–41). The IFN-γ receptor activates Jak1 and Jak2, whereas γc chain containing receptors, including the receptors for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21, use Jak1 and Jak3 (39, 42). Tyrosine phosphorylation of cytokine receptors provides binding sites for signaling molecules. The main family of DNA-binding signaling proteins with transcription activity responsible for mediating signals derived from cytokine receptors is the STAT family. STATs bind phosphorylated receptors and in turn are substrates of the Jaks. Phosphorylated STAT can interact, dimerize, traffic into the nucleus, and regulate gene expression. The mechanisms by which cytokine activation of the restricted number of single Jak or Jak combinations results in such different and specific downstream signaling events are still not understood. A large number of cytokines uses only four PTK and seven STAT proteins for their specific signaling transmission. Therefore, not only lymphocyte development, survival, and proliferation but also the outcome of an immune response by signals derived from T-helper (Th) cell lineage-defining cytokines all depend on Jak activation as initial step. Genetic analysis of Jaks in human with certain immune diseases and the generation of Jak-specific knockout (KO) mice help us to understand the exact role of Jaks in immune cell signaling.
Role of Jak family members in immune cell signaling and related diseases
Tyk2 signaling
By screening T-cell cDNA libraries, the Tyk2 gene was originally described as prototype of a new PTK gene subfamily (9, 43). Subsequently, extensive studies unraveled the functional role of Tyk2 as first non-receptor PTK of the Jak family to be essential in mediating cytokine signals. A mutant human cell line with a defect in responding to type I IFNs was transfected with a large number of different genomic DNA constructs. Type I IFN-responsiveness was restored in one transfectant, which was complemented with the Tyk2 gene (44). Thus, Tyk2 was the first Jak family member identified to be essential for cytokine signaling. Following studies investigated the downstream signaling events and transcription factors regulated by Tyk2 after IFN stimulation (4). Whereas type I IFN receptors require Tyk2 and Jak1, IFN-γ signaling depends on the combination of Tyk2 and Jak2 (39, 45). The combination of Tyk2 with Jak2 is not only required for IFN-γ signaling but also for the differentiation of IFN-γ-producing Th1 cells from naive Th cells. IL-12 is the lineage-specific cytokine responsible for Th1 generation (46, 47). IL-12 receptor (IL-12R) is associated with Tyk2 and Jak2 and activates mainly the transcription factor STAT4 (40, 48, 49). Phosphorylation of STAT4 in conjunction with signals derived from TCR stimulation induces the expression of T-bet, the master transcriptional regulator of IFN-γproducing Th1 cells (50). Interestingly, the Tyk2-associated IL-12Rβ1 chain is also used by IL-23 signaling. IL-12Rβ1 is also part of the IL-23R and associates with Tyk2, whereas the second IL-23R subunit associates with Jak2. Both IL-12 and IL-23 have common features. As heterodimeric cytokines, they share the p40 subunit and their receptors share the IL-12Rβ1 subunit. Moreover, IL-12 and IL-23 signal through Tyk2 and Jak2. But whereas Tyk2-dependent STAT4 activation is the main signaling event after IL-12 stimulation, Jak2-mediated STAT3 phosphorylation seems to be dominated by IL-23 (41, 51, 52). Thus, Tyk2 seems to be indispensible for the IFN/Th1 axis but also immune responses mediated by IL-17-producing Th17 cells, which are promoted by IL-23 may also depend on Tyk2. Since signals from numerous cytokines that are mainly involved in pro-inflammatory immune responses are mediated by Tyk2, mutations leading to loss of function of Tyk2 will be expected to lead to striking immunological phenotypes.
Consequences of Tyk2 deficiency
Since Tyk2-deficient mice are viable the consequences of Tyk2 deficiency in lymphocyte biology have been intensively studied. Lymphocyte development and proliferation are not affected in these mice, but signaling by cytokines that are important for host defense is impaired (Table 1). IL-12 signaling is strongly impaired in the absence of Tyk2, downstream activation of STAT3 and STAT4 are clearly reduced resulting in the inability of IFN-γ production by T cells. Moreover, IFN signaling itself is also dependent on functional Tyk2 expression. Both STAT3 and STAT1 activation by type I and type II IFNs are reduced in Tyk2 KO mice. Therefore, Tyk2-deficient splenocytes are impaired in their ability to respond to low concentrations of IFN-α (up to 10 U/ml), although signaling events using high concentrations of IFN-α (1000 U/ml) seem not to be affected (53). Consequently, Tyk2-deficient mice are more susceptible to viral or bacterial infections (53, 54). This susceptibility can be explained by impaired Th1 lineage development, but also Th17 differentiation seems to be affected in the absence of Tyk2. A recent experimental report showed that the production of IL-17 by γδ T cells in an infectious model of Escherichia coli was impaired in Tyk2 KO mice, without affecting IL-23 levels but IL-23-mediated IL-17 induction (55). Further studies will show to what extent IL-23-mediated maintenance of Th17 cells is Tyk2 dependent. While antigen-specific Th1 and possibly also Th17 responses that are both important for host defense are inhibited in Tyk2 KO mice, Th2 responses are enhanced. Th2 cell generation depends on IL-4 signaling that is independent from Tyk2. Th2 cell differentiation can be inhibited by Tyk2-mediated signals derived from IL-12 or IFNs. As expected, Th2-dominated diseases like allergic lung inflammation was enhanced in a mouse asthma model, using ovalbumin TCR-transgenic Tyk2-deficient T lymphocytes. Pronounced lung inflammation was due to an enhanced Th2 response with an increase of IL-4 production, increased IgE levels, and recruitment of eosinophils (56).
Table 1.
Characteristic immune phenotypes of Jak deficiencies
| Tyk2 deficiency | Jak3 deficiency | |
|---|---|---|
| Lymphocyte development and proliferation | Not affected | T cell development ↓↓ T cell proliferation ↓↓ |
| DC differentiation | IL-12 ↓, IL-23 ↓(in response to TLR4 or TLR9 activation) | Number of CD11c+ DC ↓ IL-12 ↑, IL-10 ↑(in response to TLR4 or TLR9 activation) |
| Pro-inflammatory Th cell responses | IL-12 signaling ↓ IFN signaling ↓ Susceptibility to viral infections ↑ |
IFN-g production ↓(by epigenetic modification of the IFN-g locus) |
| Th2 responses | IL-4 ↑ IgE ↑ |
IL-4 signaling ↓ |
| Phenotype in humans | Susceptibility to infections ↑ HIES-like disease |
SCID |
The only viable Jak deficiencies, Tyk2 and Jak3, show distinct immune phenotypes. Whereas in the absence of Tyk2 pro-inflammatory immune responses are impaired and Th2 responses enhanced, Jak3-deficient individuals are primarily characterized by severe immunodeficiency.
Tyk2 is not only important in effector Th cell signaling but also in dendritic cells (DCs). Adoptive transfer experiments implicated that the presence of Tyk2 in DCs is required to prime protective, IFN-γ-producing CD8+ T-cell responses, as shown by using a model of Listeria monocytogenes infection (57). A recent report focused on the role of Tyk2 in TLR-stimulated DC activation. Activation of Tyk2-deficient DCs by Toll-like receptor 9 (TLR9) or TLR4 resulted in a strongly reduced production of IL-12 and IL-23 compared to Tyk2-competent DCs (58). These findings from Tyk2-deficient DCs also contribute to explain the impaired Th1 and Th17 differentiation observed in infected Tyk2 KO mice. The exact mechanism by which Tyk2 regulates TLR-mediated signals in DCs is still unclear. Negative or positive feedback loops provided by IL-12 or IFNs may be involved. The role of type I IFNs has to be further investigated in this context to understand the findings. In addition to DC modulation by IFNs, IL-12 has been shown to activate STAT4 in DCs and macrophages, and IL-12 may induce IFN-γ production in DCs. Although the possibility of DCs to produce IFN-γ is still discussed controversially, autocrine IL-12 production has been suggested to activate DCs in a Jak2 and Tyk2-dependent manner (52, 59, 60).
Innate immune responses by natural killer (NK) cells are affected by Tyk2 deficiency. IL-18 synergizes with IL-12 to activate NK cells. Although IL-18 signaling per se is not Tyk2-dependent, the effect of IL-12 in concert with IL-18 was absent in Tyk2-deficient splenocytes. Part of the unresponsiveness of Tyk2-deficient splenocytes and NK cells was related to impairment of IL-18R expression, since IL-12-induced upregulation of IL-18R also requires Tyk2. Thus, in the absence of Tyk2, Th1 differentiation and IFN-γ production of NK cells is abrogated, while T-cell proliferation is not affected (61).
Tyk2 deficiency in humans
Only a single patient with a Tyk2 deficiency has been identified. The mutation was found to be a homozygous deletion of four nucleotides, resulting in a frame-shift and generation of a premature stop codon 5′ to the regions of Tyk2 gene that encode the major structural parts of the enzyme including the FERM, SH2, pseudokinase, and kinase domains. The patient described experienced multiple, opportunistic infections of various organs with viruses, bacteria, and fungi (62) (Table 1). Interestingly, very similar defects in cytokine signaling, as evidenced by Tyk2 KO mouse models, were observed in the patient described with Tyk2 deficiency. Absence of Tyk2 in the patients’ T cells interfered with multiple cytokine signaling pathways. Activation of STAT1, STAT2, STAT3, and STAT4 after stimulation with type I IFNs was absent. When the patients’ T cells were stimulated with IL-12 or IL-23, STAT4 activation was not detected. Moreover, IL-6 and IL-10 signaling resulted only in poor induction of SOCS3. Development of IFN-γ-producing Th1 cells was abrogated when stimulated with anti-T-cell receptor antibodies in the presence of IL-12 and anti-IL-4. The observed IFN-γ suppression was not caused by impaired T-cell activation, since Th2 cell development was strongly enhanced, even under non-polarizing cytokine conditions (62). In addition to the patients’ susceptibility to infections and impaired Th1 responses, the patient suffered from severe atopic dermatitis and elevated serum immunoglobulin E (IgE), interpreted as hyper-IgE syndrome (HIES) (Table 1). Acute atopic dermatitis and high IgE production are primarily associated with IL-4-dominated Th2 responses. Thus, the clinical and immunological observations of the described patient are in concert with the reports on impaired Th1 and enhanced Th2 responses in Tyk2 KO mice (Table 1).
More extended analysis of Tyk2 mutations in patients with HIES (or Job’s syndrome) and other diseases like atopic diseases or inflammatory autoimmune diseases are required to precise the relevance of Tyk2 mutations in human. Immune dysfunction due to Tyk2 deficiency has been reported only in one patient, and Tyk2 mutations have not been found in further patients with Job’s syndrome (63).
Independent groups have reported recently that mutations in signaling proteins downstream from Jak1 or Tyk2 are responsible for the immune defects observed in Job’s syndrome. Mutations in STAT3 are present in patients with sporadic and dominant forms of HIES (64, 65). One explanation for the increased susceptibility of patients with Job’s syndrome for infectious diseases seems to be explained by their inability for the generation of IL-17-producing Th17 cells, a pro-inflammatory Th cell lineage dependent on functional STAT3 as major driving transcription factor (66–68). The HIES-like phenotype in the described Tyk2-deficient patient might also be due to impaired Th17 responses. This could be due to impaired IL-6 and IL-23 signaling. In addition, as indicated above, Tyk2-deficient mice have a defect in IL-23 production by DCs (55, 58). Even though the clinical phenotype as well as the molecular and immunological analysis of the patient with Tyk2 deficiency was highly impressive, only one case has been reported. In contrast, a deficiency of a different Jak family member, Jak3, has been reported more frequently in humans, and the first report was published only one year after its cloning in 1994 (69–71). Jak3 deficiency is the best investigated functional Jak deficiency in humans and causes a severe phenotype that is also observed in experimental Jak3 KO mice (72–74).
Jak3 deficiency
Defects in several different genes can cause a disorder of B and T-lymphocytes known as severe combined immunodeficiency (SCID). Patients with SCID typically suffer from severe recurrent or opportunistic infections in early life. Uncontrolled progression of bacterial, viral, or fungal diseases usually result in a life-limiting outcome, with bone marrow transplantation being the lifesaving therapy. Since in early reports SCID was mainly observed in boys, the disease was linked to be X chromosome related (X-SCID). Indeed, in most cases the disease is caused due to mutations of the γc chain, encoded by the X-lined gene designated IL-2 receptor γ (IL2Rγ) (75). Phenotypically, patients with X-SCID lack T cells and NK cells. B cells are present but are impaired in function (T B+NK SCID). The finding that T-cell numbers are normal in IL-2- deficient patients and IL-2 KO mice but T cells were absent in patients with mutations of the IL-2R γ suggested that the IL-2R γ might have additional functions apart from IL-2 signaling. The IL-2R γ chain was subsequently described to be a functional component of the IL-7R and of the IL-4R (76–78). In the following years, the list of γc-containing cytokine receptors was completed by the receptors for IL-9, IL-15, and IL-21. The fact that the IL-2R γ chain is commonly used by a set of cytokines explained the severe consequences for T and NK cell development as well as the impaired function of B cells in patients with γc mutations. IL-2, IL-7, IL-9, and IL-15 are crucial for lymphocyte and NK cell proliferation and survival, whereas IL-4 and IL-21 mainly regulate B- cell function, including Ig production. Cases of non-X-linked autosomal recessive forms of T B+NK SCID suggested that other proteins that are in close functional relation to the γc chain might be involved in disease pathogenesis. Analysis of patients with autosomal recessive forms of SCID unraveled that homozygous mutations of Jak3 can cause the disease (71, 79). Since patients with Jak3-SCID have only disease limited to immune cells, transplantation of hematopoietic stem cells is the treatment of choice with high survival rates. The immune abnormalities observed in Jak3 mutated SCID patients were recapitulated by the generation of Jak3 KO mice.
Lessons from Jak3-deficient mice
All four Jak family members are crucially involved in transmitting the signals from cytokines that are responsible either for T-cell development, proliferation, or differentiation. Jak3 is the only Jak family member that is involved in all of these central functions, and therefore, lymphocyte biology is best studied in context of Jak3. In contrast to Tyk2 deletion, Jak3 deficiency results in deficits of lymphocyte development and proliferation and early studies on Jak3-deficient mice focused on these issues (Table 1). The development of T and B lymphocytes from hematopoietic progenitor cells is strictly dependent on IL-7 signaling. IL-7R is one of the γc chain-containing receptors that associate with Jak3. So it is not surprising that fetal thymus and thymi of young mice express high levels of Jak3 and Jak3-deficient mice have typically a small thymus with deficits in thymic progenitor cells (80–82). However, T cells were not totally absent but strongly inhibited in function (83, 84).
A further Jak3-associated γc chain containing receptor, IL-15R, is essential for NK cell development and intestinal γδ T cells. This explains why NK cells are absent in the periphery of Jak3-deficient mice and also in patients with Jak3 mutation-based SCID (85). Selective reconstitution of Jak3 in the thymus had no impact on B-cell development but restored T-cell development in mice. Even subpopulations like γδ T cells or NK cells that were absent in total Jak3 KO mice they reappeared after thymic Jak3 expression. Proliferation and cytokine responsiveness of peripheral Jak3-deficient T cells was still strongly impaired. Interestingly, the peripheral T cells showed an activated memory phenotype and did not respond to further activating stimuli (81, 86). Although Jak3-deficient T cells could be activated through their T-cell receptor using anti-CD3 and anti-CD28 antibodies, they failed to proliferate and in contrast were more susceptible to undergo apoptosis (87). The memory phenotype of CD4+ Th cells in Jak3 KO mice is likely the result of peripheral lymphopenia coupled to the reduction in regulatory forkhead box protein 3 (FoxP3)-expressing Th cells in Jak3 KO mice. Since IL-2 signaling and STAT5 activation cannot occur in the absence of Jak3, no CD25 or FoxP3 expression is detectable in these mice (88). The proliferation failure of Jak3-deficient CD4+ T cells is based on several features. Signaling of IL-2 and IL-7, two major cytokines involved in T-cell proliferation and homeostatic expansion, are disrupted in Jak3 KO mice. Moreover, Jak3 KO Th cells express surface markers like programmed death-1 (PD-1) and LAG-3, characterizing anergic T cells and produce anti-proliferative cytokines such as IL-10 and transforming growth factor- β (TGF-β) (88). Comparing analysis of Jak3-deficient mice with γc-deficient and Jak3/γc double KO mice unraveled their indistinguishable phenotype and also showed that Jak3 cannot be replaced by another Jak to transduce γc signals (89). Interestingly, a similar phenotype is observed by total deletion of the Jak3 downstream signaling proteins STAT5a and STAT5b (90).
Jak3 signaling influences not only T-cell development and survival but Th cell differentiation. As a γc chain-associated PTKs, Jak3 is crucial for the differentiation of classical Th2 cells. The most critical cytokine steering Th2 cell differentiation is IL-4, and IL-4 depends on Jak3 for signal transmission after binding to its γc chain containing receptor. Thus, the absence of Jak3 disrupts IL-4 signaling leading to STAT6 activation and GATA3 induction and consequently Th2 cell differentiation is blocked (Table 1). Surprisingly, Th1 cell differentiation seems also to be impaired in the absence of Jak3. In contrast to direct signaling effects, Jak3 has been suggested to influence Th1 cell differentiation by epigenetic modifications (Table 1). A very recent study reports that Jak3-mediated STAT5 activation contributes to chromatin remodeling of the IFN-γ locus. In the absence of Jak3 the binding of T-bet to the IFN-γ promoter is diminished and the generation of IFN-γ-producing Th1 cells is impaired (91). Further studies should allow better understanding the impact of Jak signaling on epigenetic modifications during Th cell lineage determination.
Given its crucial role in lymphocyte biology, it was notable that Jak3 is highly inducible in myeloid cells (92). Since myeloid and erythroid cells do not show abnormalities in Jak3 KO mice, although with time, Jak3-deficient mice develop a T-cell-mediated autoimmune disease that results in myeloid cell expansion. Although abundantly expressed, the functional role of Jak3 in DCs is less clear than the functional differences observed in Tyk2-deficient DCs (Table 1). When Jak3 KO DCs were stimulated in vitro by TLR4 or TLR9 ligands, a more pronounced production of IL-12p70 and IL-10 was observed compared to stimulated DCs from wildtype, Jak3-competent mice. These effects were even more enhanced by addition of IFN-γ during activation with TLR ligands (93). Jak3 is also involved in the enhanced production of IL12p70 by TLR-activated DCs in the presence of IL-4, a γc cytokine using Jak3 as PTK for signal transduction (94).
Jak1 deficiency
Whereas Tyk2 and Jak3 deficiencies have been investigated in human and mice, individuals with a deficiency in Jak1 have not been described. This is in accordance with the perinatal lethal phenotype of Jak1 KO mice, probably due to neurological deficits. In addition, Jak1 KO mice have major deficits in lymphopoiesis and a failure to respond to signals from class II cytokine receptors, γc cytokine receptors, and cytokine receptors that contain the gp130 subunit (95). By using a mutant cell line, U4A, the role of Jak1 in IFN signaling was evaluated. U4A cells are Jak1-deficient and do not respond to type I or type II IFNs. Transfecting U4A cells with a Jak1 construct restored tyrosine phosphorylation of Jak1 in response to IFN stimulation and allowed downstream induction of IFN-responsive genes. Moreover, these in vitro studies unraveled that only in the presence of functional Jak1 the other Jak family members Tyk2 and Jak2 can be activated by IFNs. Whereas Jak1 and Tyk2 are activated by type I IFNs, IFN-γ induces Jak1 and Jak2 activation. Since no upstream or downstream regulation was described, the combined activation of the Jak family members seems to occur at the same level within the receptor complex (39).
Jak2 deficiency
Similar to Jak1, Jak2 deficiency is lethal, and thus reports on individuals with Jak2 mutations resulting in loss of function are also missing. Generation of Jak2 KO mice led to embryonic lethality due to defective erythropoiesis. Epo receptor stimulation induces tyrosine phosphorylation of Jak2, required for the biological activity of Epo (96). Although primitive erythrocytes are found in Jak2-deleted mice, the number of c-kit Ter119+ cells is dramatically reduced, resulting in the absence of definitive erythropoiesis. Disruption of Epo signaling and signal transduction of other hormone-like cytokines that require functional Jak2 seems to be responsible for the embryonic lethality observed in Jak2 KO mice (97). Six years after the first description of conventional Jak2 KO mice with lethal phenotype, conditional Jak2 KO mice were generated (98). Surprisingly, subsequent studies concentrated only on Prl receptor signaling and the biology of mammary epithelial cells in the absence of Jak2. Thus, the functional analysis on the role of Jak2 in immune cell signaling in vivo has yet to be evaluated. In contrast to the limited data on Jak2 deficiency and its consequences for innate and adaptive immune responses, a myriad of reports exists on the consequences of Jak2 mutations leading to gain of function of this PTK and tumor development.
Jak2 mutations in human malignancies
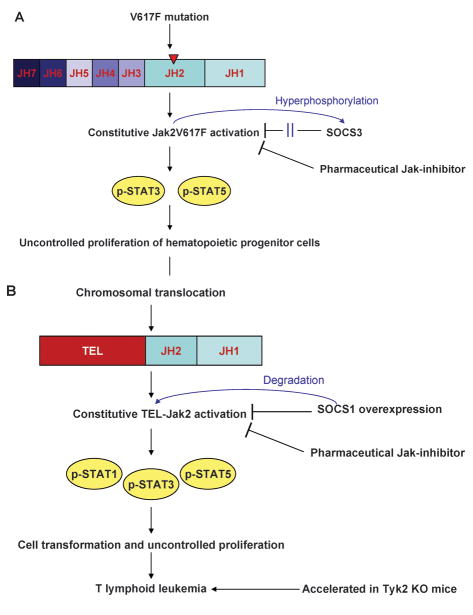
The Jak family of non-receptor PTKs is not only essential for receptor cytokine signaling during differentiation of immune responses but also mediates signals for growth, proliferation, and formation of hematopoietic cells and other tissues. Activating as well as non-activating mutations of Jaks have been described to be present in different types of hematopoietic malignancies and solid tumors. In 2005, a number of independent groups identified the importance of Jak2 in MPD (15–18). MPD make up a group of hematologic malignancies that are characterized by excess proliferation of one or more myeloid lineages. Often the disease is only manifest in a single lineage although clonal studies using X-chromosome lionization have identified that multi-lineage clonal hematopoiesis is observed in these disorders (99, 100). In contrast to acute myeloid leukemia (AML), the excess cells are differentiated and functional. MPD include primary PV, ET, primary myelofibrosis (PMF), and chronic myeloid leukemia (CML). CML has become the first cancer to be linked to a specific oncogene: BCR-Abl, in most cases caused by a reciprocal translocation of chromosomes 9 and 22 (101). In contrast, the other MPD have no association with Abl kinase activation, instead there is an association with mutations in Jak2, most commonly a V617F point mutation that results in a constitutively active kinase (102) (Fig. 2A). The V617F mutation lies within the JH2 autoinhibitory domain and this mutated kinase has been shown to autophosphorylate and become spontaneously active, rendering transfected hematopoietic cells independent of cytokines for growth and survival (15, 18), exacerbating this is the recent report that the V617F mutation may render the Jak2 protein resistant to the effect of SOCS3 binding (103) (Fig. 2A). JAK2V617F has been identified in 95% of patients with PV and over 50% of patients with ET and PMF (15–18). The very high incidence of JAK2V617F in PV may result in the disease becoming defined by the oncogenic mutation much as Abl kinase mutations define CML, with the rare cases of BCR-Abl negative CML renamed as atypical-CML.
Fig. 2. Development of hematopoietic malignancies by Jaks and possible control mechanisms.
Lymphoproliferative and myeloproliferative diseases may be caused by different Jak activating mutations (A) or fusion proteins based on chromosomal translocation (B). Both genetic changes result in constitutive activation of Jak2 and downstream STAT activation responsible for cell transformation and uncontrolled proliferation. The regulatory functions of SOCS can either not cope with the constitutive activation or are silenced by Jak2V617F hyperphosphorylation (A). The generation and use of selective Jak2 or Jak2V617F inhibitors may interrupt the signaling cascade and prevent tumor progression. When tumor-protective IFN-γ-dominated immune responses are impaired due to the absence of Tyk2, the development of TEL-Jak2-mediated T-lymphoid leukemia is accelerated in mice (B).
A number of fusion proteins comprising transcription factors and Jak2 have been recognized in lymphoproliferative and myeloproliferative disorders. Analogous to the activating JAK2V617F mutation, these fusion proteins are also constitutively activated kinases. The first fusion protein identified was TEL-Jak2, which comprises the oligomerization domain of ETS family transcription, TEL (translocated ETS leukemia), linked to the JH1 kinase domain of Jak2 (104). The fusion event is responsible for constitutive Jak2 activation. By generating TEL-Jak2 transgenic mice the striking role of unopposed Jak2 activation in the pathogenesis of T-cell leukemia was demonstrated. TEL-Jak2 transgenic mice developed a fatal leukemia, mediated by uncontrolled expansion of CD8+ T cells with activated Jak2 and downstream activation of STAT1 and STAT5 as observed in leukemic tissue (105) (Fig. 2B). Transfection of a hematopoietic pro-B cell line (Ba/F3) with TEL-Jak2 resulted in an IL-3-independent proliferation based on constitutive STAT3 and STAT5 (106). Retroviral transfection of human hematopoietic cells with a TEL-Jak2 fusion protein led to Epo-independent STAT5 activation, erythroid differentiation in vitro, and myelofibrosis in vivo upon transplantation into nonobese diabetic (NOD)/SCID mice (107). In addition to Tel-Jak2, other Jak2 fusion proteins have been noted in patients with atypical CML, including PCM1-Jak2 or BCR-Jak2 fusions. Similarly, in the setting of acute leukemia, Jak2 fusion proteins have been reported (108–111). Experimental generation of Tel-Jak1, Tel-Jak3, and Tel-Tyk2 fusion proteins also results in transformation of Ba/F3 cells by cytokine-independent activation of STAT5 and in case of Tel-Jak3 and Tel-Tyk2 also STAT1 and STAT3 (112). The oncogenic potential of PTK fusion proteins is further underlined by translocations leading to TEL-PDGFR or TEL-Abl as observed in some patients with chronic myelomonocytic leukemia or acute lymphoblastic leukemia (ALL), respectively (113, 114).
The crucial role of active Jak2 in tumor cell transformation and proliferation was underlined by kinase-targeting strategies to inhibit Jak2 activity. Retroviral expression of SOCS1 in TEL-Jak2 transformed Ba/F3 cells abrogated Jak2 activation and downstream phosphorylation of STAT1, STAT3, and STAT5. Silencing Jak2 activation by ectopic expression of SOCS1 inhibited cell proliferation and survival in vitro. More importantly, in vivo, immuno-compromised mice were protected from developing TEL-Jak2-driven tumors, when the transplanted tumor cells were retrovirally infected with SOCS1 (115). Importantly, the mutation-based constitutive activation of Jak can still be controlled by induced expression of specific Jak phosphatases such as SOCS1 or by kinase inhibitors that are currently used in clinical trials (116, 117) (Fig. 2B). In addition the TEL-Jak2 transgenic mouse model allows the interaction of Jak family members to be investigated during tumor development. Crossing TEL-Jak2 transgenic mice with Tyk2 KO mice unraveled that the onset of T-lymphoid leukemia is accelerated in the absence of Tyk2 (118) (Fig. 2B). This model shows that while one Jak family member drives malignant transformation and tumor growth, a second Jak still can limit disease progression to a certain extent by mediating cytokine signaling of IFN-γ producing cytotoxic T cells or NK cells and thus providing tumor surveillance.
Since their initial discovery, Jak2 mutations are frequently found in patients with PV, ET, PMF, and other myeloproliferative diseases. Jak2 mutations have also been reported in patients with ALL, acute myelogenous leukemia (AML), and acute megakaryoblastic leukemia (AMKL). Point mutations like V617F in Jak2 result in constitutive tyrosine phosphorylation of Jak2 and promote cell survival and proliferation independently from signals received by cytokine binding. In contrast to the numerous numbers of patients with genetic Jak2 alterations in hematological malignancies, only rare cases of point mutations of Jak3 and constitutive Jak3 activation and downstream STAT5 phosphorylation causing AMKL have been reported. Recently, the first patients with AML and Jak1 mutations have been identified, whereas Tyk2 mutations have not been described in patients with hematological malignancies (119). From published data, it appears that Jak gene mutations are rare events in solid cancer (120, 121). Interestingly, Jak3 mutations have been reported in single patients with solid cancer, i.e. breast cancer or gastric cancer (122). Large scale screening of patients are required to identify the role of Jak mutations and downstream signaling events in the pathogenesis of malignancies and immune diseases for establishing new therapeutic approaches using specific kinase inhibitors.
Pharmacological targeting of Jaks
Although at one point it was unclear whether protein kinases would be therapeutically useful targets, there are now eight PTK inhibitors (imatinib, gefitinib, dasatinib, sunitinib, erlotinib, nilotinib, lapatinib, sorafenib) approved by the Food and Drug Administration (FDA) for the therapy of several malignancies, including myeloproliferative as well as lymphoproliferative diseases and solid cancers. The first FDA-approved kinase inhibitor, imatinib, an inhibitor of Abl kinase, is indicated for the treatment of CML (123). However, it is now clear that imatinib also inhibits other unrelated tyrosine kinases. This function is advantageous, in that it is efficacious in the treatment of solid cancers such as gastrointestinal stromal tumors and breast cancer. Other PTK inhibitors already approved or used in current clinical trials target the EGFR, vascular endothelial growth factor receptor, platelet-derived growth factor receptor, FLT-3, and Src family kinases. Yet, others are known to inhibit activity of multiple PTK as well as serine/threonine kinases. Interestingly, even kinase inhibitors with less specificity and inhibitory activity on multiple protein kinases (e.g. dasatinib and sunitinib) show clinical efficacy with tolerable toxicity. PTK inhibitors are even being tested in combination (e.g. dasatinib and imatinib or dasatinib and erlotinib).
Targeting Jak3
Conceptually, all Jak family members might be targets in different settings; however, given the very limited function of Jak3, as exemplified in Jak3-SCID patients, the first efforts to generate Jak-selective inhibitors for therapeutic use focused on this kinase. Several advantages favor Jak3 as reasonable target to generate new immunosuppressive agents. In contrast to the other members of the Jak family, Jak3 expression appears to be most highly expressed in hematopoietic cells. Not only is Jak3 expression limited but also Jak3 function seems to be quite restricted due to its selective association with cytokine receptors. Among the Jak family members, Jak3 has an unique characteristic in that it only associates with one receptor, the γc chain, a subunit of the receptors for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21. As a result, Jak3 mutations in human or germline deletion of Jak3 in mice result in immunodeficiency but not other abnormalities. Consequently, deficits are only present within lymphoid cells but have not been described in other organs.
Ten years ago, the quinazoline derivatives WHI-P131 and WHI-P154 were initially noted to have activity against glioblastoma, presumably due to activity against the EGFR (124). These inhibitors were also thought to have activity against Jak3 kinase activity (125), but in retrospect the compounds were neither selective nor potent Jak3 inhibitors (126). Other compounds like tyrphostin AG490 or PNU156804 have been reported to inhibit Jak3 (127, 128). However, their selectivity has not been firmly established.
Potent and reasonably selective Jak3 inhibitors have been developed. Furthest along in development and clinical testing is the compound CP-690,550. A recent independent study showed that CP-690,550 has high affinity for Jak3, with little binding to unrelated kinases (129). The in vivo effect of CP-690,550 was first investigated in animal models of organ graft rejection. Efficacy could be shown for preventing heart or kidney rejection after transplantation without observing metabolic abnormalities or severe side effects due to immunosuppression (130). Marked reduction in lymphocyte subsets was observed in a dose-dependent manner but was transient since after dosing cessation lymphocyte numbers began to normalize (131). In cynomolgus monkeys, oral dosing of Jak3 mainly reduced numbers of NK cells and effector memory CD8+ T cells (132). Prevention of graft rejection and prolongation of kidney allograft survival in cynomolgus monkeys has been suggested to be related to the decrease of NK cells and T cells and lower production levels of IFN-γ in CP-690,550-treated animals (133, 134). Jak3 inhibition with this compound was also effective in a transplantation setup in rodents and prevented allograft vasculopathy of transplanted aortas in rats (135). In addition to the effects of Jak3 inhibition in transplantation models, CP-690,550 was recently shown to be effective in inflammatory diseases in rodents. Jak3 inhibition also prevented cartilage damage in a model of collagen-induced arthritis in mice and adjuvant-induced arthritis in rats. The main immunologic effect observed in the animals with experimental rheumatoid arthritis was the reduction of IL-6 production when treated with CP-690,550 (136). The exact mechanism by which Jak3 inhibition inhibits IL-6 production, a critical cytokine that drives inflammatory destruction in rheumatoid arthritis, remains to be determined.
Since Jak3 affects the γc cytokine IL-4, it was reasonable to test CP-690,550 in asthma. In a mouse model of Th2-mediated asthma, treatment with CP-690,550 could effectively inhibit pulmonary eosinophilia. IL-4 is the central cytokine in this model of pulmonary disease and is dependent on functional association of Jak3 to the γc chain-containing IL-4R. CP-690,550 abrogated IL-4-mediated signals and inhibited IL-13, eotaxin, and the eosinophilic influx into the lungs (137).
CP-690,550 is under current investigation in phase II clinical trials for the therapy of rheumatoid arthritis, psoriasis, and the prevention of renal transplant rejection. Preliminary data from first trials are promising, showing efficacy with acceptable toxicity. In a phase II study for rheumatoid arthritis, 70% to 81% of patients responded with an ACR20 improvement compared to 29% in the placebo group. ACR70 was achieved by 13 to 28% in the CP-690,550 group, whereas only in 3% of the patients in the placebo cohort an ACR70 response was observed (138). Notably, these responses were achieved in patients that failed with standard of care therapies including methotrexate or biologics like tumor necrosis factor antagonists. Similarly, in psoriasis, a significant and dose-dependent reduction of inflammation and scaling was observed as measured by a modified PASI score (139). A first phase I dose escalation study of CP-690,550 has also been performed in the setting of kidney transplantation. Twenty-eight patients were included in the study, 22 received CP-690,550 divided in different groups of 5 mg BID, 15 mg BID, and 30 mg BID, with 6 patients were in the placebo group. No graft loss was reported. At present there are more than a dozen clinical trials underway testing CP 690,550 in rheumatoid arthritis, psoriasis, and renal transplantation.
Although Jak3 is crucial for lymphocyte survival and proliferation and Jak3 deficiency results in severe lymphopenia in mice and human, no changes of the major CD4+ or CD8+ T-lymphocyte subsets have been observed in clinical studies. Consistent with preclinical studies, a decrease of the absolute CD16+ and CD56+ NK cell numbers has been observed in patients receiving CP-690,550 therapy (140).
An important adverse event in the study of renal transplantation was an increased incidence of infections, although in this setting, the patients also received other immunosuppressive drugs. Laboratory parameters also revealed a significant reduction of hemoglobin concentration in the low and high dose group compared to the patients receiving placebo (140). In this study, no major changes were observed in total or relative numbers of neutrophils, lymphocytes, eosinophils, basohphils, monocytes, or platelets. In contrast, dose-dependent neutropenia was noted in the rheumatoid arthritis study. A likely explanation for the effects of CP-690,550 on hemoglobulin concentrations and neutrophil counts might be related to the effect of this drug on Jak2. As discussed above, Jak2 signaling is important for Epo, although the importance of this kinase for granulocyte colony-stimulating factor signaling is less clear (38). In vitro, CP-690,550 has been reported to have considerable affinity for Jak2 and to a lesser extent for Tyk2 (129). The ability of CP-690,550 to inhibit these other Jaks will be an important issue to study in the future. Because of the attractiveness of Jak3 as a target, a variety of companies have generated Jak3 inhibitors, which are at different levels of development. These include Rigel (R-348, Phase I), Pharmacopeia/Wyeth (PS-608504, preclinical), Vertex (VX-509, preclinical), and Cytopia/Novartis (preclinical).
Targeting Jak2 in MPD
The dramatic discovery that the mutations of Jak2 underlie nearly all cases of PV and many cases of ET and MPD provided a rationale for the use of Jak2 inhibitors in this setting (15). At present, there are at least 15 clinical trials underway using various PTK inhibitors in the setting of MPD. Several companies have generated putatively selective Jak2 inhibitors, which are being tested in these disorders including S*BIO (SB1518), Exelixis (XL019), Incyte (INCB018424), TargeGen (TG101348), and Cephalon (lestaurtinib) (141, 142). The extent to which these compounds are truly Jak2 selective needs to be independently assessed. With respect to this issue, it is notable that the Incyte compound has been reported to inhibit both Jak1 and Jak2. Despite the fact that germline deletion of Jak1 and Jak2 are embryonically lethal, INCB018424 is presently being studied in rheumatoid arthritis and psoriasis. Moreover, the drug is also being tested in prostate cancer, multiple myeloma, AML, and CML. From all these trials, we will learn much about the consequences of inhibiting Jaks in human and the necessity for achieving specificity.
Lestaurtinib (CEP-701) is FDA designated as orphan drug for AML, which was originally thought to target FLT-3 and TrkA. However, lestaurtinib has also been reported to inhibit Jak2 and is therefore being tested in patients with Jak2 mutations (143). Furthemore, dasatinib, a PTK inhibitor approved for use in CML, is an inhibitor of Src family PTKs and BCR-Abl. Dasatinib was not efficacious in an animal model of Jak2V617F-induced PV, but in vitro dasatinib was able to inhibit myeloid and erythroid colony growth of peripheral blood cells from PV patients (144, 145). A recent analysis showed that at least at high doses (1μM), dasatinib can inhibit Jak2 activity in vitro (129). How dasatinib inhibits Jak2V617F-driven proliferation is unclear, but clinical trials are underway. Finally, although imatinib has little activity towards Jak2, efficacy of imatinib is currently tested in phase II clinical trials for PV. It is very clear that the importance of selectively inhibiting PTKs is a moving target.
CP-690,550 has been reported to preferentially inhibit the signaling pathways activated by mutated Jak2 (146). Interestingly, the inhibition observed with CP-690,550 on mutated Jak2 cells was more pronounced than in cells expressing wildtype Jak2. In vitro, CP-690,550 at concentrations of 1μM induced apoptosis in erythroid progenitor cells of patients with PV but not from healthy controls (146). The nanomolar binding capacity of CP-690,550 to Jak2 is very similar to the activity against the main target Jak3, and clinical trials have to show the efficacy of this primary Jak3 inhibitor in PV (129).
Concluding remarks
During the last two decades, remarkable advances have been made in the field of cytokine biology, starting with the elucidation of the principal pathways of cytokine signaling and culminating in the transfer from basic science to the introduction of new therapeutic drugs. The generation and investigation of KO mice as well as intensive observations from several human diseases have helped to delineate the signaling pathways that derive from cytokine stimulation. Loss-of-function mutations of Jak3 are responsible for a subgroup of SCID patients and a patient with a HIES-like syndrome caused by Tyk2 deficiency has been described. Gain-of-function mutations of Jak2 and Jak2 fusion proteins are responsible for a number of lymphoproliferative and myeloproliferative diseases. In the past decade, we have identified the structure of Jak proteins, and this has led to our improved understanding of the regulatory mechanisms that control these kinases including the JH domains, phosphorylation sites, and associated regulatory proteins. However, we are still quite ignorant about the detailed structure of Jaks and many aspects of the cell biology of Jaks and their cognate receptors. Much remains to be learned about how Jaks transduce signals from numerous cytokine receptors and how they are regulated. Nonetheless, numerous clinical trials using Jak and other PTK inhibitors are underway and a great deal of new information about the consequence of inhibiting Jaks in humans is likely to be revealed in the near future.
References
- 1.Venter JC, et al. The sequence of the human genome. Science. 2001;291:1304–51. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 2.Rane SG, Reddy EP. JAK3: a novel JAK kinase associated with terminal differentiation of hematopoietic cells. Oncogene. 1994;9:2415–23. [PubMed] [Google Scholar]
- 3.Yamaoka K, Saharinen P, Pesu M, Holt VE, 3rd, Silvennoinen O, O’Shea JJ. The Janus kinases (Jaks) Genome Biol. 2004;5:253. doi: 10.1186/gb-2004-5-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–21. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 5.Ihle JN, Witthuhn BA, Quelle FW, Yamamoto K, Silvennoinen O. Signaling through the hematopoietic cytokine receptors. Annu Rev Immunol. 1995;13:369–98. doi: 10.1146/annurev.iy.13.040195.002101. [DOI] [PubMed] [Google Scholar]
- 6.Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 7.Liu KD, Gaffen SL, Goldsmith MA. JAK/STAT signaling by cytokine receptors. Curr Opin Immunol. 1998;10:271–8. doi: 10.1016/s0952-7915(98)80165-9. [DOI] [PubMed] [Google Scholar]
- 8.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–62. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 9.Firmbach-Kraft I, Byers M, Shows T, Dalla-Favera R, Krolewski JJ. tyk2, prototype of a novel class of non-receptor tyrosine kinase genes. Oncogene. 1990;5:1329–36. [PubMed] [Google Scholar]
- 10.Riedy MC, et al. Genomic sequence, organization, and chromosomal localization of human JAK3. Genomics. 1996;37:57–61. doi: 10.1006/geno.1996.0520. [DOI] [PubMed] [Google Scholar]
- 11.Pritchard MA, Baker E, Callen DF, Sutherland GR, Wilks AF. Two members of the JAK family of protein tyrosine kinases map to chromosomes 1p31.3 and 9p24. Mamm Genome. 1992;3:36–8. doi: 10.1007/BF00355839. [DOI] [PubMed] [Google Scholar]
- 12.Luo H, et al. Mutation in the Jak kinase JH2 domain hyperactivates Drosophila and mammalian Jak-Stat pathways. Mol Cell Biol. 1997;17:1562–71. doi: 10.1128/mcb.17.3.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saharinen P, Silvennoinen O. The pseudokinase domain is required for suppression of basal activity of Jak2 and Jak3 tyrosine kinases and for cytokine-inducible activation of signal transduction. J Biol Chem. 2002;277:47954–63. doi: 10.1074/jbc.M205156200. [DOI] [PubMed] [Google Scholar]
- 14.Chen M, et al. Complex effects of naturally occurring mutations in the JAK3 pseudokinase domain: evidence for interactions between the kinase and pseudokinase domains. Mol Cell Biol. 2000;20:947–56. doi: 10.1128/mcb.20.3.947-956.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–8. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 16.Kralovics R, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 17.Baxter EJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 18.Levine RL, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–97. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Bellanne-Chantelot C, et al. Genetic and clinical implications of the Val617Phe JAK2 mutation in 72 families with myeloproliferative disorders. Blood. 2006;108:346–52. doi: 10.1182/blood-2005-12-4852. [DOI] [PubMed] [Google Scholar]
- 20.Zhou YJ, et al. Unexpected effects of FERM domain mutations on catalytic activity of Jak3: structural implication for Janus kinases. Mol Cell. 2001;8:959–69. doi: 10.1016/s1097-2765(01)00398-7. [DOI] [PubMed] [Google Scholar]
- 21.Chen M, et al. The amino terminus of JAK3 is necessary and sufficient for binding to the common gamma chain and confers the ability to transmit interleukin 2-mediated signals. Proc Natl Acad Sci U S A. 1997;94:6910–5. doi: 10.1073/pnas.94.13.6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haan S, et al. Dual role of the Jak1 FERM and kinase domains in cytokine receptor binding and in stimulation-dependent Jak activation. J Immunol. 2008;180:998–1007. doi: 10.4049/jimmunol.180.2.998. [DOI] [PubMed] [Google Scholar]
- 23.Funakoshi-Tago M, Pelletier S, Moritake H, Parganas E, Ihle JN. Jak2 FERM domain interaction with the erythropoietin receptor regulates Jak2 kinase activity. Mol Cell Biol. 2008;28:1792–801. doi: 10.1128/MCB.01447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radtke S, et al. The Jak1 SH2 domain does not fulfill a classical SH2 function in Jak/STAT signaling but plays a structural role for receptor interaction and up-regulation of receptor surface expression. J Biol Chem. 2005;280:25760–8. doi: 10.1074/jbc.M500822200. [DOI] [PubMed] [Google Scholar]
- 25.Kurzer JH, Argetsinger LS, Zhou YJ, Kouadio JL, O’Shea JJ, Carter-Su C. Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-B beta. Mol Cell Biol. 2004;24:4557–70. doi: 10.1128/MCB.24.10.4557-4570.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou YJ, et al. Distinct tyrosine phosphorylation sites in JAK3 kinase domain positively and negatively regulate its enzymatic activity. Proc Natl Acad Sci U S A. 1997;94:13850–5. doi: 10.1073/pnas.94.25.13850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng H, Ross JA, Frost JA, Kirken RA. Phosphorylation of human Jak3 at tyrosines 904 and 939 positively regulates its activity. Mol Cell Biol. 2008;28:2271–82. doi: 10.1128/MCB.01789-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Funakoshi-Tago M, Tago K, Kasahara T, Parganas E, Ihle JN. Negative regulation of Jak2 by its auto-phosphorylation at tyrosine 913 via the Epo signaling pathway. Cell Signal. 2008 doi: 10.1016/j.cellsig.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Funakoshi-Tago M, Pelletier S, Matsuda T, Parganas E, Ihle JN. Receptor specific downregulation of cytokine signaling by autophosphorylation in the FERM domain of Jak2. EMBO J. 2006;25:4763–72. doi: 10.1038/sj.emboj.7601365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiao H, Berrada K, Yang W, Tabrizi M, Platanias LC, Yi T. Direct association with and dephosphorylation of Jak2 kinase by the SH2-domain-containing protein tyrosine phosphatase SHP-1. Mol Cell Biol. 1996;16:6985–92. doi: 10.1128/mcb.16.12.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Brien KB, O’Shea JJ, Carter-Su C. SH2-B family members differentially regulate JAK family tyrosine kinases. J Biol Chem. 2002;277:8673–81. doi: 10.1074/jbc.M109165200. [DOI] [PubMed] [Google Scholar]
- 32.Tong W, Zhang J, Lodish HF. Lnk inhibits erythropoiesis and Epo-dependent JAK2 activation and downstream signaling pathways. Blood. 2005;105:4604–12. doi: 10.1182/blood-2004-10-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishida-Takahashi R, et al. Phosphorylation of Jak2 on Ser(523) inhibits Jak2-dependent leptin receptor signaling. Mol Cell Biol. 2006;26:4063–73. doi: 10.1128/MCB.01589-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazurkiewicz-Munoz AM, et al. Phosphorylation of JAK2 at serine 523: a negative regulator of JAK2 that is stimulated by growth hormone and epidermal growth factor. Mol Cell Biol. 2006;26:4052–62. doi: 10.1128/MCB.01591-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–65. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 36.Johnston JA, O’Shea JJ. Matching SOCS with function. Nat Immunol. 2003;4:507–9. doi: 10.1038/ni0603-507. [DOI] [PubMed] [Google Scholar]
- 37.Argetsinger LS, et al. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993;74:237–44. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- 38.Parganas E, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–95. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 39.Muller M, et al. The protein tyrosine kinase JAK1 complements defects in interferon- alpha/beta and -gamma signal transduction. Nature. 1993;366:129–35. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]
- 40.Bacon CM, McVicar DW, Ortaldo JR, Rees RC, O’Shea JJ, Johnston JA. Interleukin 12 (IL-12) induces tyrosine phosphorylation of JAK2 and TYK2: differential use of Janus family tyrosine kinases by IL-2 and IL-12. J Exp Med. 1995;181:399–404. doi: 10.1084/jem.181.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parham C, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 42.Johnston JA, et al. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994;370:151–3. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- 43.Krolewski JJ, Lee R, Eddy R, Shows TB, Dalla-Favera R. Identification and chromosomal mapping of new human tyrosine kinase genes. Oncogene. 1990;5:277–82. [PubMed] [Google Scholar]
- 44.Velazquez L, Fellous M, Stark GR, Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992;70:313–22. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- 45.Watling D, et al. Complementation by the protein tyrosine kinase JAK2 of a mutant cell line defective in the interferon-gamma signal transduction pathway. Nature. 1993;366:166–70. doi: 10.1038/366166a0. [DOI] [PubMed] [Google Scholar]
- 46.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 47.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 48.Bacon CM, et al. Interleukin 12 induces tyrosine phosphorylation and activation of STAT4 in human lymphocytes. Proc Natl Acad Sci U S A. 1995;92:7307–11. doi: 10.1073/pnas.92.16.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobson NG, et al. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. J Exp Med. 1995;181:1755–62. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 51.Cho ML, et al. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006;176:5652–61. doi: 10.4049/jimmunol.176.9.5652. [DOI] [PubMed] [Google Scholar]
- 52.Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O’Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139–56. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 53.Shimoda K, et al. Tyk2 plays a restricted role in IFN alpha signaling, although it is required for IL-12-mediated T cell function. Immunity. 2000;13:561–71. doi: 10.1016/s1074-7613(00)00055-8. [DOI] [PubMed] [Google Scholar]
- 54.Karaghiosoff M, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549–60. doi: 10.1016/s1074-7613(00)00054-6. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura R, Shibata K, Yamada H, Shimoda K, Nakayama K, Yoshikai Y. Tyk2-signaling plays an important role in host defense against Escherichia coli through IL-23-induced IL-17 production by gammadelta T cells. J Immunol. 2008;181:2071–5. doi: 10.4049/jimmunol.181.3.2071. [DOI] [PubMed] [Google Scholar]
- 56.Seto Y, et al. Enhanced Th2 cell-mediated allergic inflammation in Tyk2-deficient mice. J Immunol. 2003;170:1077–83. doi: 10.4049/jimmunol.170.2.1077. [DOI] [PubMed] [Google Scholar]
- 57.Aizu K, et al. An important role of Tyk2 in APC function of dendritic cells for priming CD8+ T cells producing IFN-gamma. Eur J Immunol. 2006;36:3060–70. doi: 10.1002/eji.200636173. [DOI] [PubMed] [Google Scholar]
- 58.Tokumasa N, et al. Expression of Tyk2 in dendritic cells is required for IL-12, IL-23, and IFN-gamma production and the induction of Th1 cell differentiation. Blood. 2007;110:553–60. doi: 10.1182/blood-2006-11-059246. [DOI] [PubMed] [Google Scholar]
- 59.Fukao T, Frucht DM, Yap G, Gadina M, O’Shea JJ, Koyasu S. Inducible expression of Stat4 in dendritic cells and macrophages and its critical role in innate and adaptive immune responses. J Immunol. 2001;166:4446–55. doi: 10.4049/jimmunol.166.7.4446. [DOI] [PubMed] [Google Scholar]
- 60.Frucht DM, Fukao T, Bogdan C, Schindler H, O’Shea JJ, Koyasu S. IFN-gamma production by antigen-presenting cells: mechanisms emerge. Trends Immunol. 2001;22:556–60. doi: 10.1016/s1471-4906(01)02005-1. [DOI] [PubMed] [Google Scholar]
- 61.Shimoda K, et al. Partial impairment of interleukin-12 (IL-12) and IL-18 signaling in Tyk2- deficient mice. Blood. 2002;99:2094–9. doi: 10.1182/blood.v99.6.2094. [DOI] [PubMed] [Google Scholar]
- 62.Minegishi Y, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25:745–55. doi: 10.1016/j.immuni.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 63.Woellner C, et al. The hyper IgE syndrome and mutations in TYK2. Immunity. 2007;26:535. doi: 10.1016/j.immuni.2007.05.007. author reply 6. [DOI] [PubMed] [Google Scholar]
- 64.Holland SM, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–19. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 65.Minegishi Y, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–62. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 66.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–81. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 67.Laurence A, O’Shea JJ. T(H)-17 differentiation: of mice and men. Nat Immunol. 2007;8:903–5. doi: 10.1038/ni0907-903. [DOI] [PubMed] [Google Scholar]
- 68.Milner JD, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–6. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kawamura M, et al. Molecular cloning of L-JAK, a Janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes. Proc Natl Acad Sci U S A. 1994;91:6374–8. doi: 10.1073/pnas.91.14.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takahashi T, Shirasawa T. Molecular cloning of rat JAK3, a novel member of the JAK family of protein tyrosine kinases. FEBS Lett. 1994;342:124–8. doi: 10.1016/0014-5793(94)80485-0. [DOI] [PubMed] [Google Scholar]
- 71.Macchi P, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–8. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 72.Ihle JN. The Janus protein tyrosine kinases in hematopoietic cytokine signaling. Semin Immunol. 1995;7:247–54. doi: 10.1006/smim.1995.0029. [DOI] [PubMed] [Google Scholar]
- 73.Thomis DC, Berg LJ. The role of Jak3 in lymphoid development, activation, and signaling. Curr Opin Immunol. 1997;9:541–7. doi: 10.1016/s0952-7915(97)80108-2. [DOI] [PubMed] [Google Scholar]
- 74.Pesu M, Candotti F, Husa M, Hofmann SR, Notarangelo LD, O’Shea JJ. Jak3, severe combined immunodeficiency, and a new class of immunosuppressive drugs. Immunol Rev. 2005;203:127–42. doi: 10.1111/j.0105-2896.2005.00220.x. [DOI] [PubMed] [Google Scholar]
- 75.Noguchi M, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–57. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 76.Noguchi M, et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science. 1993;262:1877–80. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- 77.Kondo M, et al. Functional participation of the IL-2 receptor gamma chain in IL-7 receptor complexes. Science. 1994;263:1453–4. doi: 10.1126/science.8128231. [DOI] [PubMed] [Google Scholar]
- 78.Kondo M, et al. Sharing of the interleukin-2 (IL-2) receptor gamma chain between receptors for IL-2 and IL-4. Science. 1993;262:1874–7. doi: 10.1126/science.8266076. [DOI] [PubMed] [Google Scholar]
- 79.Russell SM, et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 80.Gurniak CB, Berg LJ. Murine JAK3 is preferentially expressed in hematopoietic tissues and lymphocyte precursor cells. Blood. 1996;87:3151–60. [PubMed] [Google Scholar]
- 81.Thomis DC, Berg LJ. Peripheral expression of Jak3 is required to maintain T lymphocyte function. J Exp Med. 1997;185:197–206. doi: 10.1084/jem.185.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baird AM, Lucas JA, Berg LJ. A profound deficiency in thymic progenitor cells in mice lacking Jak3. J Immunol. 2000;165:3680–8. doi: 10.4049/jimmunol.165.7.3680. [DOI] [PubMed] [Google Scholar]
- 83.Thomis DC, Gurniak CB, Tivol E, Sharpe AH, Berg LJ. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science. 1995;270:794–7. doi: 10.1126/science.270.5237.794. [DOI] [PubMed] [Google Scholar]
- 84.Nosaka T, et al. Defective lymphoid development in mice lacking Jak3. Science. 1995;270:800–2. doi: 10.1126/science.270.5237.800. [DOI] [PubMed] [Google Scholar]
- 85.Park SY, et al. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity. 1995;3:771–82. doi: 10.1016/1074-7613(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 86.Saijo K, Park SY, Ishida Y, Arase H, Saito T. Crucial role of Jak3 in negative selection of self-reactive T cells. J Exp Med. 1997;185:351–6. doi: 10.1084/jem.185.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thomis DC, Lee W, Berg LJ. T cells from Jak3-deficient mice have intact TCR signaling, but increased apoptosis. J Immunol. 1997;159:4708–19. [PubMed] [Google Scholar]
- 88.Mayack SR, Berg LJ. Cutting edge: an alternative pathway of CD4+ T cell differentiation is induced following activation in the absence of gamma-chain-dependent cytokine signals. J Immunol. 2006;176:2059–63. doi: 10.4049/jimmunol.176.4.2059. [DOI] [PubMed] [Google Scholar]
- 89.Suzuki K, Nakajima H, Saito Y, Saito T, Leonard WJ, Iwamoto I. Janus kinase 3 (Jak3) is essential for common cytokine receptor gamma chain (gamma(c))-dependent signaling: comparative analysis of gamma(c), Jak3, and gamma(c) and Jak3 double-deficient mice. Int Immunol. 2000;12:123–32. doi: 10.1093/intimm/12.2.123. [DOI] [PubMed] [Google Scholar]
- 90.Yao Z, et al. Stat5a/b are essential for normal lymphoid development and differentiation. Proc Natl Acad Sci U S A. 2006;103:1000–5. doi: 10.1073/pnas.0507350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi M, Lin TH, Appell KC, Berg LJ. Janus-kinase-3-dependent signals induce chromatin remodeling at the Ifng locus during T helper 1 cell differentiation. Immunity. 2008;28:763–73. doi: 10.1016/j.immuni.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Musso T, et al. Regulation of JAK3 expression in human monocytes: phosphorylation in response to interleukins 2, 4, and 7. J Exp Med. 1995;181:1425–31. doi: 10.1084/jem.181.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamaoka K, Min B, Zhou YJ, Paul WE, O’Shea JJ. Jak3 negatively regulates dendritic- cell cytokine production and survival. Blood. 2005;106:3227–33. doi: 10.1182/blood-2005-02-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lutz MB, et al. Differential functions of IL-4 receptor types I and II for dendritic cell maturation and IL-12 production and their dependency on GM-CSF. J Immunol. 2002;169:3574–80. doi: 10.4049/jimmunol.169.7.3574. [DOI] [PubMed] [Google Scholar]
- 95.Rodig SJ, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373–83. doi: 10.1016/s0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 96.Witthuhn BA, et al. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74:227–36. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- 97.Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 98.Krempler A, Qi Y, Triplett AA, Zhu J, Rui H, Wagner KU. Generation of a conditional knockout allele for the Janus kinase 2 (Jak2) gene in mice. Genesis. 2004;40:52–7. doi: 10.1002/gene.20063. [DOI] [PubMed] [Google Scholar]
- 99.Fialkow PJ, Gartler SM, Yoshida A. Clonal origin of chronic myelocytic leukemia in man. Proc Natl Acad Sci U S A. 1967;58:1468–71. doi: 10.1073/pnas.58.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adamson JW, Fialkow PJ, Murphy S, Prchal JF, Steinmann L. Polycythemia vera: stem- cell and probable clonal origin of the disease. N Engl J Med. 1976;295:913–6. doi: 10.1056/NEJM197610212951702. [DOI] [PubMed] [Google Scholar]
- 101.Nowell PC. Discovery of the Philadelphia chromosome: a personal perspective. J Clin Invest. 2007;117:2033–5. doi: 10.1172/JCI31771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao R, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280:22788–92. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hookham MB, et al. The myeloproliferative disorder-associated JAK2 V617F mutant escapes negative regulation by suppressor of cytokine signaling 3. Blood. 2007;109:4924–9. doi: 10.1182/blood-2006-08-039735. [DOI] [PubMed] [Google Scholar]
- 104.Lacronique V, et al. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278:1309–12. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- 105.Carron C, et al. TEL-JAK2 transgenic mice develop T-cell leukemia. Blood. 2000;95:3891–9. [PubMed] [Google Scholar]
- 106.Spiekermann K, Pau M, Schwab R, Schmieja K, Franzrahe S, Hiddemann W. Constitutive activation of STAT3 and STAT5 is induced by leukemic fusion proteins with protein tyrosine kinase activity and is sufficient for transformation of hematopoietic precursor cells. Exp Hematol. 2002;30:262–71. doi: 10.1016/s0301-472x(01)00787-1. [DOI] [PubMed] [Google Scholar]
- 107.Kennedy JA, et al. Expression of TEL-JAK2 in primary human hematopoietic cells drives erythropoietin-independent erythropoiesis and induces myelofibrosis in vivo. Proc Natl Acad Sci U S A. 2006;103:16930–5. doi: 10.1073/pnas.0604902103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reiter A, et al. The t(8;9)(p22;p24) is a recurrent abnormality in chronic and acute leukemia that fuses PCM1 to JAK2. Cancer Res. 2005;65:2662–7. doi: 10.1158/0008-5472.CAN-04-4263. [DOI] [PubMed] [Google Scholar]
- 109.Bousquet M, et al. The t(8;9)(p22;p24) translocation in atypical chronic myeloid leukaemia yields a new PCM1-JAK2 fusion gene. Oncogene. 2005;24:7248–52. doi: 10.1038/sj.onc.1208850. [DOI] [PubMed] [Google Scholar]
- 110.Griesinger F, et al. A BCR-JAK2 fusion gene as the result of a t(9;22)(p24;q11.2) translocation in a patient with a clinically typical chronic myeloid leukemia. Genes Chromosomes Cancer. 2005;44:329–33. doi: 10.1002/gcc.20235. [DOI] [PubMed] [Google Scholar]
- 111.Cirmena G, et al. A BCR-JAK2 fusion gene as the result of a t(9;22)(p24;q11) in a patient with acute myeloid leukemia. Cancer Genet Cytogenet. 2008;183:105–8. doi: 10.1016/j.cancergencyto.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 112.Lacronique V, et al. Transforming properties of chimeric TEL-JAK proteins in Ba/F3 cells. Blood. 2000;95:2076–83. [PubMed] [Google Scholar]
- 113.Golub TR, Barker GF, Lovett M, Gilliland DG. Fusion of PDGF receptor beta to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell. 1994;77:307–16. doi: 10.1016/0092-8674(94)90322-0. [DOI] [PubMed] [Google Scholar]
- 114.Janssen JW, et al. The fusion of TEL and ABL in human acute lymphoblastic leukaemia is a rare event. Br J Haematol. 1995;90:222–4. doi: 10.1111/j.1365-2141.1995.tb03407.x. [DOI] [PubMed] [Google Scholar]
- 115.Rottapel R, et al. The tumor suppressor activity of SOCS-1. Oncogene. 2002;21:4351– 62. doi: 10.1038/sj.onc.1205537. [DOI] [PubMed] [Google Scholar]
- 116.Kamizono S, et al. The SOCS box of SOCS-1 accelerates ubiquitin-dependent proteolysis of TEL-JAK2. J Biol Chem. 2001;276:12530–8. doi: 10.1074/jbc.M010074200. [DOI] [PubMed] [Google Scholar]
- 117.Frantsve J, Schwaller J, Sternberg DW, Kutok J, Gilliland DG. Socs-1 inhibits TEL-JAK2-mediated transformation of hematopoietic cells through inhibition of JAK2 kinase activity and induction of proteasome-mediated degradation. Mol Cell Biol. 2001;21:3547–57. doi: 10.1128/MCB.21.10.3547-3557.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stoiber D, et al. TYK2 is a key regulator of the surveillance of B lymphoid tumors. J Clin Invest. 2004;114:1650–8. doi: 10.1172/JCI22315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xiang Z, et al. Identification of somatic JAK1 mutations in patients with acute myeloid leukemia. Blood. 2008;111:4809–12. doi: 10.1182/blood-2007-05-090308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bardelli A, et al. Mutational analysis of the tyrosine kinome in colorectal cancers. Science. 2003;300:949. doi: 10.1126/science.1082596. [DOI] [PubMed] [Google Scholar]
- 121.Greenman C, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–8. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jeong EG, et al. Somatic mutations of JAK1 and JAK3 in acute leukemias and solid cancers. Clin Cancer Res. 2008;14:3716–21. doi: 10.1158/1078-0432.CCR-07-4839. [DOI] [PubMed] [Google Scholar]
- 123.Druker BJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 124.Narla RK, Liu XP, Myers DE, Uckun FM. 4-(3′-Bromo-4′hydroxylphenyl)-amino-6,7-dimethoxyquinazoline: a novel quinazoline derivative with potent cytotoxic activity against human glioblastoma cells. Clin Cancer Res. 1998;4:1405–14. [PubMed] [Google Scholar]
- 125.Sudbeck EA, et al. Structure-based design of specific inhibitors of Janus kinase 3 as apoptosis-inducing antileukemic agents. Clin Cancer Res. 1999;5:1569–82. [PubMed] [Google Scholar]
- 126.Changelian PS, et al. The specificity of JAK3 kinase inhibitors. Blood. 2008;111:2155–7. doi: 10.1182/blood-2007-09-115030. [DOI] [PubMed] [Google Scholar]
- 127.Nielsen M, et al. Constitutive activation of a slowly migrating isoform of Stat3 in mycosis fungoides: tyrphostin AG490 inhibits Stat3 activation and growth of mycosis fungoides tumor cell lines. Proc Natl Acad Sci U S A. 1997;94:6764–9. doi: 10.1073/pnas.94.13.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stepkowski SM, et al. Selective inhibitor of Janus tyrosine kinase 3, PNU156804, prolongs allograft survival and acts synergistically with cyclosporine but additively with rapamycin. Blood. 2002;99:680–9. doi: 10.1182/blood.v99.2.680. [DOI] [PubMed] [Google Scholar]
- 129.Karaman MW, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–32. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 130.Changelian PS, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302:875–8. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- 131.Kudlacz E, et al. The novel JAK-3 inhibitor CP-690550 is a potent immunosuppressive agent in various murine models. Am J Transplant. 2004;4:51–7. doi: 10.1046/j.1600-6143.2003.00281.x. [DOI] [PubMed] [Google Scholar]
- 132.Conklyn M, Andresen C, Changelian P, Kudlacz E. The JAK3 inhibitor CP-690550 selectively reduces NK and CD8+ cell numbers in cynomolgus monkey blood following chronic oral dosing. J Leukoc Biol. 2004;76:1248–55. doi: 10.1189/jlb.0504282. [DOI] [PubMed] [Google Scholar]
- 133.Borie DC, et al. Immunosuppression by the JAK3 inhibitor CP-690,550 delays rejection and significantly prolongs kidney allograft survival in nonhuman primates. Transplantation. 2005;79:791–801. doi: 10.1097/01.tp.0000157117.30290.6f. [DOI] [PubMed] [Google Scholar]
- 134.Paniagua R, et al. Effects of JAK3 inhibition with CP-690,550 on immune cell populations and their functions in nonhuman primate recipients of kidney allografts. Transplantation. 2005;80:1283–92. doi: 10.1097/01.tp.0000177643.05739.cd. [DOI] [PubMed] [Google Scholar]
- 135.Rousvoal G, et al. Janus kinase 3 inhibition with CP-690,550 prevents allograft vasculopathy. Transpl Int. 2006;19:1014–21. doi: 10.1111/j.1432-2277.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 136.Milici AJ, Kudlacz EM, Audoly L, Zwillich S, Changelian P. Cartilage preservation by inhibition of Janus kinase 3 in two rodent models of rheumatoid arthritis. Arthritis Res Ther. 2008;10:R14. doi: 10.1186/ar2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kudlacz E, Conklyn M, Andresen C, Whitney-Pickett C, Changelian P. The JAK-3 inhibitor CP-690550 is a potent anti-inflammatory agent in a murine model of pulmonary eosinophilia. Eur J Pharmacol. 2008;582:154–61. doi: 10.1016/j.ejphar.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 138.Kremer JM, Bloom BJ, Breedveld FC, et al. A randomized, double-blind placebo-controlled trial of 3 dose levels of CP-690, 550 versus placebo in the treatment of active rheumatoid arthritis. Arthritis Rheum. 2006;54:S36–836. [Google Scholar]
- 139.Chan G, Cunshan W, Boy M, Chow V, Herron J. Dose-dependent reduction in psoriasis severity as evidence of immunosuppressive activity of an oral Jak3 inhibitor in humans. Am J Transplant. 2006;6(Suppl 2):87. [Google Scholar]
- 140.van Gurp E, et al. Phase 1 dose-escalation study of CP-690 550 in stable renal allograft recipients: preliminary findings of safety, tolerability, effects on lymphocyte subsets and pharmacokinetics. Am J Transplant. 2008;8:1711–8. doi: 10.1111/j.1600-6143.2008.02307.x. [DOI] [PubMed] [Google Scholar]
- 141.Geron I, et al. Selective inhibition of JAK2-driven erythroid differentiation of polycythemia vera progenitors. Cancer Cell. 2008;13:321–30. doi: 10.1016/j.ccr.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 142.Wernig G, et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell. 2008;13:311–20. doi: 10.1016/j.ccr.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 143.Hexner EO, et al. Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood. 2008;111:5663–71. doi: 10.1182/blood-2007-04-083402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zaleskas VM, et al. Molecular pathogenesis and therapy of polycythemia induced in mice by JAK2 V617F. PLoS ONE. 2006;1:e18. doi: 10.1371/journal.pone.0000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wappl M, et al. Dasatinib inhibits progenitor cell proliferation from polycythaemia vera. Eur J Clin Invest. 2008;38:578–84. doi: 10.1111/j.1365-2362.2008.01982.x. [DOI] [PubMed] [Google Scholar]
- 146.Manshouri T, et al. The JAK kinase inhibitor CP-690,550 suppresses the growth of human polycythemia vera cells carrying the JAK2V617F mutation. Cancer Sci. 2008;99:1265–73. doi: 10.1111/j.1349-7006.2008.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]