Abstract
The combination of materials design and advances in nanotechnology has led to the development of new therapeutic protein delivery systems. The pulmonary, nasal, buccal and other routes have been investigated as delivery options for protein therapy, but none result in improved patient compliances and patient quality of life as the oral route. For the oral administration of these new systems, an understanding of protein transport is essential because of the dynamic nature of the gastrointestinal tract and the barriers to transport that exist.
Models have been developed to describe the transport between the gastrointestinal lumen and the bloodstream, and laboratory techniques like cell culture provide a means to investigate the absorption and transport of many therapeutic agents. Biomaterials, including stimuli-sensitive complexation hydrogels, have been investigated as promising carriers for oral delivery. However, the need to develop models that accurately predict protein blood concentration as a function of the material structure and properties still exists.
INTRODUCTION
Recent advances in the discovery and delivery of drugs to cure chronic diseases are achieved by the combination of intelligent material design with advances in nanotechnology. Since many drugs act as protagonists or antagonists to different chemicals in the body, a delivery system that can respond to the concentrations of certain molecules in the body is invaluable. For this purpose, intelligent therapeutics or “smart drug delivery” calls for the design of the newest generation of sensitive materials based on molecular recognition. In this effort, biomimetic carriers are the main materials for such applications.
Biomimetic polymeric networks can be prepared by designing interactions between the building blocks of biocompatible networks and the desired specific ligands and by stabilizing these interactions by a three-dimensional structure. These structures are at the same time flexible enough to allow for diffusion of solvent and ligand into and out of the networks. These artificial materials can be used as unique systems or incorporated into existing drug delivery technologies that can aid in the delivery of biomolecules and restore the desired profiles of naturally occurring compounds in the body.
In recent years, there has been considerable work in preparing materials and finding new uses for nanoscale structures based on biomaterials. Uses such as carriers for controlled and targeted drug delivery, micropatterned devices, systems for biological recognition, have shown the versatility of these biopolymeric materials as indicated by Langer and Peppas (2003). We believe that the development of nanoparticulate systems for drug delivery applications has taken a level of sophistication never before seen in the field of drug delivery (Ponchel and Irache, 1998).
This new level of sophistication has led to the successful investigation of various drug delivery routes that are advantageous over the oft-used administration route of injection. In this review, the benefits and challenges of these routes and work with therapeutic protein delivery via these routes will be covered. Particular focus is given to the oral delivery route as it is recognized to be a favored delivery option for patients and for the unique challenges presented by the varying and complex nature of the organs in the gastrointestinal tract. Laboratory techniques and a mathematical model for analyzing absorption and transport, as well as tactics for improving the absorption and transport properties of therapeutic agents, are presented. The review closes with a look at several types of biomaterials and how the unique structures of these materials can improve the absorption and transport of therapeutic proteins.
DRUG DELIVERY ROUTES
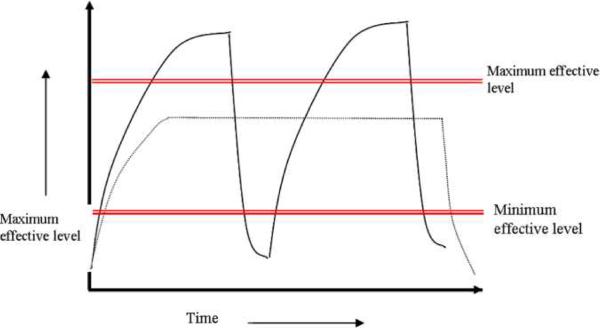
The goal of controlled drug delivery devices is to achieve the desired therapeutic effect over a long period of time. With traditional tablets or injections, the drug levels in the blood rise after administration and then decrease until the next one (See Figure 1). A problem with this profile is that sometimes the drug levels in the blood either achieve toxic levels or ineffective levels. In controlled drug delivery systems designed for long term administration, the drug level in the blood follows a profile in which the drug level remains constant, between the effective maximum and minimum, for an extended period of time. Sustained released systems are more patient friendly and also offer the advantages of increased effectiveness and cost effectiveness.
Figure 1.
Drug levels in the blood with (—) traditional repeated dosing and (----) controlled delivery dosing.
Therapeutic proteins are presently delivered by injections. In some cases, in order to have a better control, multiple daily injections are necessary. However, this is not enough if the quality of lives of these patients is not improved. To eliminate the pain (Partanen and Rissanen, 2000) and fear (Nir et al., 2003) associated with injections and increase patient compliance, researchers have actively worked to identify administration routes or alternatives that are painless and non-invasive. The mucosal layers of the body provide access to the bloodstream and are often marked by high surface areas for absorption. The use of transmucosal delivery has many advantages, however each layer presents unique challenges for the delivery of proteins.
Pulmonary Delivery
Pulmonary delivery is an attractive site for non-invasive drug delivery because of the large surface area – approximately 140 m2 – for drug absorption (Owens et al., 2003). The epithelial cells of the alveoli are very thin so there are minimal barriers to transport. First-pass metabolism is bypassed and minimal enzymatic activity of note occurs within the lungs. The alveoli can be targeted when the drug is delivered as an aerosol as long as the mass median aerodynamic diameter is less than 5 μm (Agu et al., 2001). Factors that affect the use of the pulmonary route include lung disease, smoking, exercise, and the necessity to control the breathing rate (Owens et al., 2003).
Nasal Delivery
Nasal delivery has been investigated as an alternative delivery method due to its high levels of epithelial layer decoration and subepithelial vascularization. Similar to pulmonary delivery, nasal delivery avoids first-pass metabolism (Türker et al., 2004) and experiences lower enzymatic activity when compared to the gastrointestinal tract and liver (Ugwoke et al., 2001). Furthermore, an additional advantage of nasal delivery is the ease of self-medication, directly increasing patient compliance.
Problems with the use of the nasal route include the nose's filtration of foreign particulates and rapid mucociliary clearance, both of which affect the absorption potential for macromolecules (Kissel and Werner, 1998). Other considerations like nasal secretions and the pH of the nasal cavity must be accounted for as it is important to not adversely affect the physiological functions and conditions of the nasal cavity (Arora et al., 2002). The low molecular weight cutoff of the nasal route (~1000 Da) (Ugwoke et al., 2001) often requires the use of permeation enhancers, which can lead to nasal irritation and transport of harmful pathogens across the mucosa.
Buccal Delivery
The buccal/oral mucosa is favorable due to its highly vascularized nature and large surface area for absorption. Delivery via the buccal mucosa bypasses first pass metabolism in the liver and could lead to better drug bioavailability. The presence and variable flow rate of saliva acts as a barrier to drug transport, though. The buccal cavity acts like skin in that it is a first line of defense against foreign agents (Nicolazzo et al., 2005), but many buccal strategies researched rely on permeation enhancers to facilitate transport (Song et al., 2004) which may allow for the absorption of undesired entities like viruses due to the susceptibility of the mouth to airborne pathogens.
Alternative Delivery Sites
Researchers like Kopeček et al. (1992) and Brønsted and Kopeček (1992) have investigated the delivery of proteins, protected by hydrogels, by using the colon as their target area because of the low activity of proteolytic enzymes. Additional delivery sites that have been explored for their accessibility include the rectal, vaginal and ocular mucosas. Protein delivery via the rectal mucosa has been investigated by Uchida et al. (1999). An advantage of rectal delivery is the avoidance of enzymatic degradation since there can be direct protein absorption by the lymphatic system (Owens et al., 2003). This approach has the disadvantages of bacterial activity, interference from fecal material, and longer disintegration and dissolution times in the colon milieu (Fix, 1996). Use of the vaginal mucosa for protein delivery has been met with localized adverse reactions and low, variable absorption (Owens et al., 2003); furthermore, the non-universality of the route limits its capability to be used for protein delivery needs that are common for both genders. The ocular route provides rapid systemic absorption but contains peptidases that can destroy proteins. The irritation caused by the addition of a foreign molecule to the eye can also trigger tear formation, leading to lower bioavailabilities (Sinha and Trehan, 2003).
Oral Delivery
In the United States, the preferred route of drug administration is the oral route as patients prefer this method for the ease of self-administration and associated convenience and lower costs (Ruppar, 2008). The oral route is not without its challenges, though. The gastrointestinal tract is a complicated, dynamic system of organs with a primary goal of breaking down food for the body's daily needs. The proteolytic enzymes and low permeabilities found within the gastrointestinal tract are just some of the limitations faced when utilizing the oral route for native drug delivery. Many peptides and proteins will be destroyed prior to being absorbed in the small intestine. In vivo studies of native insulin in a murine model have shown bioavailabilities via the oral route of less than 1% (Perakslis et al., 2007). With a proper understanding of the anatomy and physiology of the gastrointestinal tract, certain delivery systems and strategies can be utilized to improve on these bioavailabilities and lead to the realization of the true potential of utilizing the oral route for protein delivery.
TRANSPORT AND ABSORPTION
The development of novel carriers and improved drug delivery profiles requires a multi-pronged approach to fully assess the viability of improving the absorption and transport of therapeutic agents. One such approach is mathematical modeling. Models allow for the analysis of many iterations before proceeding to development and experimentation, which can reduce operating costs. Mathematical models can also be used in conjunction with laboratory experiments to quantify transport rates and investigate correlations to the animal and human scale. Cell culture remains one of the most valuable and widely used laboratory techniques for investigating protein absorption and transport.
Transport and Absorption Model for Proteins in the Gastrointestinal Tract
To analyze the transport of proteins in the gastrointestinal environment, one must utilize models with appropriate boundary conditions to represent the gastrointestinal lumen and the bloodstream. One such model, developed by our laboratory to describe this transport, utilizes the basic conservation equation for protein absorption across the intestinal wall represented in Equation (1):
| (1) |
where c is the concentration of a freely diffusing protein, ci is the intracellular concentration of the protein, D is the effective protein diffusivity, ν is the superficial filtrate-flow velocity, ε is the intestinal wall porosity, P is the intestinal cell permeability coefficient and r1 is the rate of protein binding to the intestinal wall.
For a bound protein, this rate is expressed by Equation (2):
| (2) |
where r1 is the rate of protein binding.
When the protein is intracellular, the rate is expressed by Equation (3):
| (3) |
where r2 is the rate of protein intracellular metabolism.
Protein uptake may be expressed as a linear function, as shown in Equation (4)–(5):
| (4) |
| (5) |
The boundary conditions for this model are shown in Equation (6) for the gastrointestinal side (x=0) and Equation (7) for the blood side (x=L):
| (6) |
| (7) |
where RG is the phenomenological rejection coefficient at the GI side, KG is the vesicular mass transfer coefficient at the GI side, RB is the phenomenological rejection coefficient at the blood side, and KB is the vesicular mass transfer coefficient at the blood side. Ranges of values for the aforementioned parameters may be seen in Table 1.
Table 1.
Range of experimental values of theoretical parameters used in gastrointestinal transport models
| Parameter | Value |
|---|---|
| D (cm2/s) | 1 × 10−9 to 1 × 10−11 |
| ν (mL/cm2h) | 1 × 10−2 to 1 × 10−3 |
| KG | 1 × 10−7 to 1 × 10−9 |
| KB | 1 × 10−8 to 1 × 10−9 |
| ε | 0.42 |
| RG | 0.3 to 1 |
| RB | 0 to 0.7 |
Assessing Drug Transport via Cell Culture
Several approaches have been used for the study of drug permeation, including transport through isotropic, lipophilic phases or animal studies. When screening for permeation characteristics, the choice of test system always represents a compromise between high throughput with low predictive potential and low throughput with high predictive potential (Braun et al., 2000). Cell cultures correspond to the intermediate level of complexity of these permeation studies.
Approaches to the study of drug transport and permeation involve transport through preparations of intestinal tissue such as everted sac, everted rings, and intestinal loops, among others. However, these preparations have several disadvantages such as the lack of cellular metabolism. This may limit their utilization in the study of active transport processes. Also, membrane isolation processes may damage the membrane's potential to carry out enzymatic or carrier functions.
Cell cultures offer many advantages over conventional techniques (Audus et al., 1990) including: (a) rapid assessment of the potential permeability and metabolism of a drug; (b) opportunity to elucidate the transport mechanism and pathway for a drug; (c) rapid evaluation of strategies to achieve drug targeting, enhance drug transport and minimize drug degradation; (d) opportunity to use human, rather than, animal tissue; and (e) opportunity to minimize time-consuming and expensive animal studies.
An important factor for consideration when performing cell studies is the utilization of the correct microporous membrane (e.g. polycarbonate, nitrocellulose) as a support. This membrane should support cell attachment and growth. It should also be sufficiently translucent so that the monolayer can be monitored by microscopic techniques. And finally, it should be permeable to hydrophilic and hydrophobic solutes of different molecular weights.
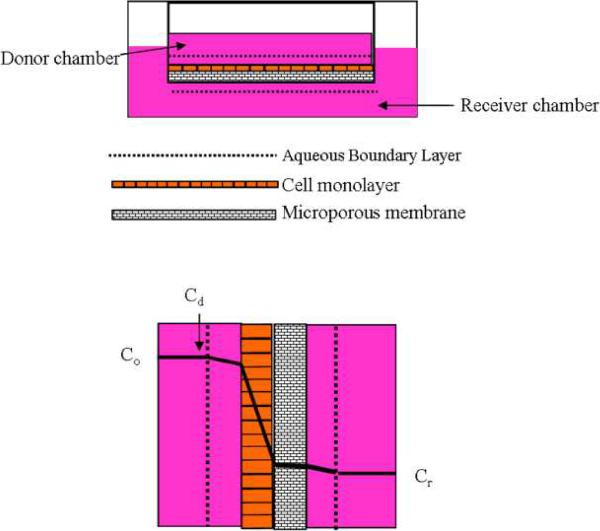
Obviously the ideal diffusion characteristics of a cell culture model are observed when the cell monolayer, not the microporous membrane, provides the major diffusion barrier (See Figure 2). Therefore, the selection of the correct pore size, surface area and physiochemical properties of the membrane play an important role when performing the cell studies.
Figure 2.
Potential barriers to solute transport in a cell culture system.
(A) Cell monolayer system grown onto a microporous system.
(B) Concentration profile system for the solute with the largest concentration drop within the cell monolayer.
Co, original concentration of the solute, Cd, concentration of the solute in the donor chamber, Cr, concentration of the solute in the receiver chamber
The selection of the cell line is very important in order to mimic the biological barrier with an in vitro cell culture system. Different human colon carcinoma cell lines (i.e. Caco-2, HT-29, SW116, LS147T, SW-480) have been considered for the screening of different drugs. However, Caco-2 and HT-29 have been the most investigated because of their ability to express morphologic features of mature enterocytes or goblet cells.
Transport of Drugs Through Caco-2 Cell Monolayers
The use of the Caco-2 cell culture as an intestinal epithelial model has dramatically increased in recent years. The Caco-2 cell culture model is a human-derived colon adenocarcinoma cell line. Confluent monolayers of Caco-2 cells exhibit morphological and functional similarities to the small intestinal epithelium. These characteristics have made this cell culture model a very useful tool for the assessment of drug permeabilities and transport through the epithelial cell monolayer.
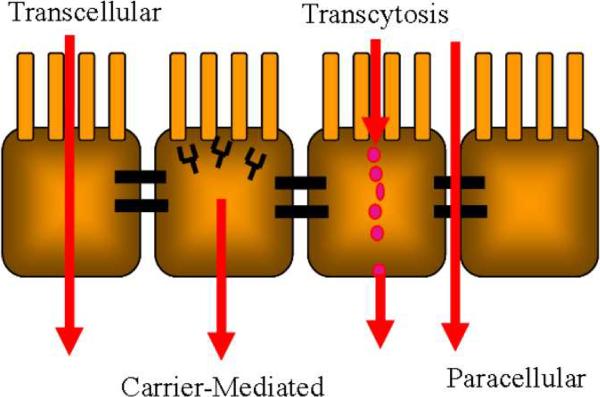
The two primary pathways for molecules to cross the epithelium and reach the bloodstream are the paracellular route and transcellular route. A pictorial depiction of these routes may be found in Figure 3. Paracellular transport involves the movement of the molecule of interest between the epithelial cells whereas transcellular transport occurs when the molecule passes through the epithelial cell. Hydrophilic and charged molecules primarily use the paracellular route; however, the size constraints presented by the tight junctions limit this route to molecules of a size less than 11 Å (Fasano, 1998). This means that only small molecules can pass through the tight junctions. Most drugs cannot pass freely unless the tight junctions are disrupted.
Figure 3.
Transport pathways through the cell monolayer
Three mechanisms that utilize the transcellular route exist. The first of these mechanisms is transcellular passive transport. This mechanism is often reserved for small, neutral molecules that pass through the layer via diffusion without hindrance. The second mechanism, transcytosis, occurs when a molecule in the proximity of the cell membrane is entrapped in a lipid bilayer known as a vesicle. The vesicle passes into the cell and will either release its contents through the basolateral side of the cell, achieving transcellular transport, or release it within the cell for digestion. Carrier-mediated transport is the third transcellular mechanism and occurs when a molecule reversibly binds to complexes in the lipid bilayer. The complex dissociates within the cell and the molecule passes to the basolateral side, forms a new complex with the bilayer, and exits the cell (Blanchette et al., 2004).
Caco-2 monolayers have been used to study drug transport via the transcellular and paracellular transport routes. They have been shown to be excellent models for drugs that are transported through the passive transcellular pathway (Artursson et al., 2001; Pade and Stavchansky, 1998). When studying drugs that are transported through the paracellular route, low permeabilities have been observed when compared with in vivo data. However, these differences are of quantitative rather than qualitative nature (Artursson, 1990).
Artursson (1990) investigated the paracellular permeability of different compounds with different molecular weights. They found that the permeabilities decreased with molecular weight in a comparable fashion in both Caco-2 monolayers and human intestine in vivo. However, the permeability values from the monolayers were almost 100-fold lower than the values obtained in vivo. From these results they suggest that there are fewer pores in the tight junctions of the Caco-2 monolayers, but that the average pore diameters are comparable in both models. Results obtained by Tanaka et al. (1995) also support this hypothesis.
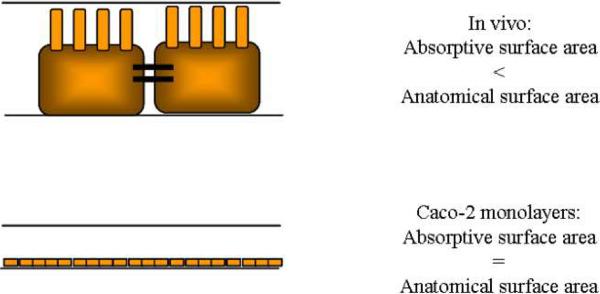
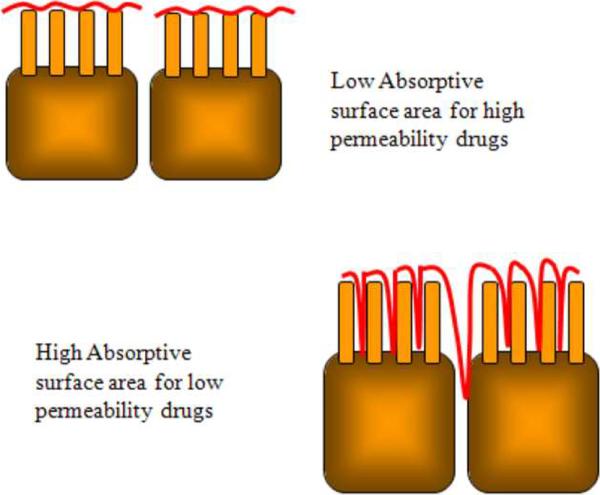
Another explanation to the low permeability data is based on the differences in the absorptive surface area between the human jejunum and Caco-2 monolayers (Artursson et al., 2001). In vivo absorptive surface area is smaller than the anatomical surface area but in the Caco-2 monolayers, both the absorptive and anatomical area are assumed to be equal (See Figure 4). Drugs with high permeabilities are rapidly and completely absorbed through the villus tips. Permeabilities of these drugs are very comparable in both systems. Conversely, drugs with low permeabilities will remain longer in the intestinal lumen before they are absorbed. Therefore, they may diffuse further down the length of the villi (See Figure 5). This diffusion would have to take into account the anatomical surface area resulting in higher permeabilities in vivo than in the Caco-2 monolayers. A third explanation for the low paracellular permeabilities involves the differences in the regulation of tight junction permeability and paracellular water fluxes in the cell monolayer as compared to intestinal tissues in situ (Artursson et al., 2001).
Figure 4.
Comparison of absorptive surface areas for drugs in the intestine in vivo and in Caco-2 monolayers.
Figure 5.
Comparison of absorptive surface areas for high and low permeability drugs.
The tight junction is a cell-cell junction that seals adjacent epithelial cells together, preventing dissolved molecules in the extracellular medium from passing from one side of the epithelial sheet to the other. Tight junctions are located at the luminal aspect of adjacent epithelial cells. These structures were physiologically and microscopically defined during 1960's and 1970's. But it was not until 1986 that Zonula Occludens-1, ZO-1, the first tight junction protein, was identified (Gan et al., 1998). Now there are more than two dozen known proteins that are associated in the junction complex.
The absorption of large hydrophilic molecules is limited to the paracellular pathway. In turn, this pathway is restricted because of the presence of the tight junctions. Different approaches have been investigated in order to induce the paracellular transport of large molecules, such as proteins, by disrupting the tight junctions. Some of these approaches include the use of surfactants (Nerukar et al., 1996), H2-antagonists (Gan et al., 1998), polysaccharides such as chitosans (Schipper et al., 1996; Lueβen et al., 1997), and poly(acrylic acid) derivatives such as polycarbonil (Lueβen et al., 1997), among others (Hurni et al., 1993; Fasano and Uzzau, 1997; Lee et al., 1991). However, some of these approaches compromise the integrity of the cell monolayer in their effort of permeability enhancement (Swenson and Curatolo, 1992).
Different factors affect the paracellular permeability of drugs. Knipp et al. (1997) found that the effect of electrical charge on the permeation of the solutes seemed to decrease with increasing molecular radius. They also found that the absence of Ca2+ on the culture medium allowed the transport of solutes through the paracellular route by affecting the integrity of the tight junctions. The effect of other divalent cations such as Mg2+ and Ba2+ were also tried resulting in non-equivalents for Ca2+. Furthermore, studies carried out by Cogburn et al (Cogburn et al., 1991) indicated that the paracellular permeabilities decreased with lowered temperatures.
BARRIERS TO ORAL DRUG DELIVERY
Several chemical and physical barriers exist that limit the delivery of proteins via the oral route. The gastrointestinal (GI) tract has many functions including food digestion, water and nutrient absorption, waste elimination and immune protection capabilities. As a system, the GI tract is comprised of many organs, but the stomach, small intestine and large intestine are of primary interest when considering oral drug delivery.
The safe and effective oral administration of peptides and proteins is not an easy task to achieve due to their complex chemistry. Moreover, the success in these delivery systems strongly depends on simultaneously addressing issues like poor permeability, degradation, rapid clearance, and chemical and conformational stability. In the gastrointestinal tract, it is important to consider physiological factors like residence time, pH, and ionic strength as they play a major role in the successful absorption and transport of therapeutic agents and directly correspond to the aforementioned issues.
The stomach is responsible for digestion. The transit of food through the stomach occurs over 1–3 h (Hwang et al., 1998) and has a drastic effect on the gastric pH level. In the fasted state, the gastric pH is approximately 2; in the fed state, this value can go as high as 6.5 for brief periods (Klausner et al., 2003). The gastric juices that aid in digestion have an ionic strength of 0.1–0.165 mM (Kararli, 1995).
The small intestine is responsible for the bulk of nutrient absorption into the bloodstream. By length, the duodenum is 20–30 cm long; the jejunum, 2.5 m; the ileum, 3.5 m (Granger et al., 1985). The small intestinal transit time is around 3–4 h (Kararli, 1995) with the duodenal time limited to approximately one minute (Hwang et al., 1998). Pancreatic juices are secreted into the small intestine in the duodenum in part to raise the pH of the digested food following gastric emptying. The ionic strength of the pancreatic juices is 0.119–0.190 mM. The pH of the small intestine ranges between 6–7 and remains relatively constant in the presence of food (Kararli, 1995).
Of primary interest is the presence of anatomical features in the small intestine that enhance the surface area to facilitate absorption. These features are the Kerckring mucosal folds; the villi, which are finger-like projections lined with enterocytes (columnar absorbing cells) (Kararli, 1995); and the microvilli, which are present on the enterocytes. All told, the presence of these features yields a small intestinal surface area approximately the size of a basketball court (Read and Sugden, 1988). The microvilli combine to form a brush border that must be crossed in order for nutrients and water to reach the bloodstream.
Perhaps the most formidable chemical barrier to the oral delivery of therapeutic proteins is the presence of proteolytic, degradative enzymes in the gastrointestinal tract. Proteolytic enzymes are present to break dietary proteins down into their amino acid constituents for absorption, but a therapeutic protein must be absorbed intact in order to preserve its pharmacological activity. The work of proteolytic enzymes begins in the stomach with the presence of pepsin, but the final breakdown of the proteins occurs in the small intestine. The pancreatic juices secreted into the small intestine contain trypsin, chymotryspin, elastases and lipases, along with other enzymes that work to deconstruct the protein. Additionally, membrane-bound enzymes like peptidases are present on the small intestinal brush border (Johnson, 2001).
Physical barriers to protein absorption include an aqueous unstirred layer on the epithelial cells and the tight junctions formed between the enterocytes. The aqueous layer consists of water, mucus and the glycocalyx. The mucus layer consists of glycoproteins that help to stabilize the layer and prohibit absorption. Additionally, the presence of these secretions can lead to interactions between the solute and the layer that limit absorption of the drug (Larhed et al., 1997). Tight junctions exist between the epithelial cells and limit paracellular transport of macromolecules. The dimensions of these openings have been estimated to be on the order of 3–10 Å, allowing for good permeability values for small molecules but low values for proteins (Hamman et al., 2005).
ABSORPTION ENHANCEMENT STRATEGIES
While the epithelium of the small intestine is more permeable than the mucosa of the buccal cavity (Nicolazzo et al., 2005), the bioavailability of proteins introduced via the oral route is often too low to justify using the route as an effective therapeutic alternative. The reasons for this include the proteolytic enzymes and the physicochemical barriers introduced by the intestinal epithelium (see also models presented above). Protease inhibitors and permeation enhancers can be used to compensate for these barriers and enhance absorption. Additionally, utilizing a system to protect the drug from degradation in the enzymatic environment to deliver the maximum payload to the absorption site is another strategy that will be covered in a later section.
Protease Inhibitors
Protease inhibitors have been investigated as a means to enhance absorption by curbing the proteolytic activity of the degradative enzymes in the gastrointestinal tract. Bernkop-Schnürch has prepared a review of select inhibitors and the protease they affect (Bernkop-Schnurch, 1998). Non-amino acid based inhibitors, modified peptides and polypeptide inhibitors are common examples of these materials. Many have challenged the inclusion of supplemental protease inhibitors in drug delivery formulations due to their potential side effects. The inhibition of digestive enzymes may assist in protecting the therapeutic protein, but it also retards the digestion of proteins consumed through one's diet. The presence of the inhibitors leads to the secretion of more proteases by the pancreas, ultimately leading to hypertrophy and hyperplasia of the organ. While strategies like simultaneous protein-inhibitor release to force localized action and inhibitor immobilization on a delivery system have been considered, the potential for systemic toxicity and mucosal damage limit the viability of these compounds to be used for absorption enhancement.
Permeation Enhancers
Permeation enhancers are compounds that temporarily disrupt the epithelial barrier and tight junctions to increase the permeability of drugs or molecules and allow for greater quantities to reach the bloodstream. An ideal enhancer would be non-toxic and biocompatible; additionally, it should act at the moment the drug is at the absorption site and have a reversible action to limit or prevent damage to the epithelial layer. A review of permeation enhancer varieties has been prepared by Aungst (2000). Classifications of common permeation enhancers include calcium chelators, which enhance paracellular transport by disrupting the tight junctions, and surfactants, which enhance transcellular transport by disrupting the lipid bilayer (Salama et al., 2006). Chitosans are also considered to be effective enhancers, but they, like the previous classes, have been shown to cause damage to the epithelial integrity and function (Salama et al., 2004). An additional concern with permeation enhancers is the enhanced permeation indiscriminately allowing for toxins and pathogens to enter the bloodstream (Carino and Mathiowitz, 1999). The ability to introduce undesired toxins and permanently disrupt the epithelium suggests that permeation enhancers are not the ideal choice for increasing drug absorption.
Non-specific Targeting Using Mucoadhesion
It is believed that improvements of local absorption are possible if the carrier has the ability to have prolonged contact with the mucus or cells. Mucoadhesion is a method to improve polymer interaction, either chemical or physical, with a mucus layer. The term mucoadhesion can be classified as a form of bioadhesion, which is the occurrence of a bond between two biological surfaces or a biological surface with a synthetic surface. Mucoadhesion is an important concept to consider when developing a drug delivery system for the oral route because increased interactions between a carrier and the intestinal mucosa will improve the residence time within the body and, by having intimate contact between a drug loaded carrier and the mucosa, eliminate some of the barriers for drug absorption (Robinson, 1989).
Five theories exist to explain the muco- or bioadhesive nature of some materials: electronic, wetting, fracture, adsorption, and diffusion (Peppas et al., 1984). The electronic theory suggests that a double layer of electrical charge develops at the interface of two materials with different electronic structures – in this case, the polymer and the mucus. Attractive forces across this layer lead to the adhesion. The wetting theory is based on the ability of one of the materials of interest to spread and form intimate contact with the other. The fracture theory is applicable when studying mucoadhesive properties by mechanical measurements as it is based on the forces required to pull two surfaces apart.
The adsorption theory is the most widely accepted of the five theories and states that mucoadhesion occurs due to secondary forces like hydrogen bonds. The interaction between polymeric carriers and the glycoproteins of the intestinal mucosa is thought to occur via hydrogen bonding and other secondary forces like van der Waals interactions and ionic bonds (Chickering and Mathiowitz, 1999). Anionic polymers have been shown to have better bioadhesion than cationic or neutral polymers (Salamat-Miller et al., 2005). For this reason, materials like poly(acrylic acid), poly(methacrylic acid), and other carboxyl and hydroxyl group bearing polymers have been used for mucoadhesive systems (Peppas, 2007). The diffusion theory is related to the interpenetration and entanglement of polymer chains with the mucus layer to form semipermanent bonds. The depth to which this penetration occurs is thought to have a direct impact on the strength of the bond, with penetrations of 0.2–0.5 μm being effective. This theory can be used to explain mucoadhesive properties seen with tethered structure like poly(ethylene glycol) grafts.
Many accept that it is not strictly one theory that defines the mucoadhesive properties of a system, but a combination of several theories (Dodou et al., 2005). As a polymer carrier starts to swell, it will expose more of its surface and be able to have more direct contact with the mucus layer (wetting theory). The swelling of a material by taking in fluid from the mucus is very important in the formation of a mucoadhesive bond (Grabovac et al., 2005). As the material comes into contact with the mucosa, secondary forces like hydrogen bonds can form to attach the two surfaces (electronic and adsorption). Depending on the structure of the material, interpenetration may occur between the two surfaces to further strengthen the bond (diffusion).
DELIVERY OF THERAPEUTIC PROTEINS
The number and frequency of protein therapeutics used in medical treatments is at an all time high, and this should only continue to increase as further scientific discoveries are made. At present, the United States Food and Drug Administration has approved more than 130 proteins and peptides for therapeutic purposes. These proteins are often naturally synthesized in the body and highly specific, so there are fewer adverse effects with protein therapeutics than synthesized small molecule drugs (Leader et al., 2008). The absorption and transport of these proteins is highly dependent on characteristics like size (i.e. molecular weight and hydrodynamic radius) and ionic nature (isoelectric point).
Insulin
Insulin is a hormone produced by the body to regulate blood glucose levels so that one does not enter a state of hyperglycemia, which is the presence of an excess of glucose in circulation. The insulin molecule has a molecular weight of 5808 Da and an isoelectric point of 5.3–5.5. Monomeric insulin has a hydrodynamic radius of 12–13 Å whereas hexameric insulin, a common form of insulin in the presence of zinc, has a hydrodynamic radius of 28 Å (Hovgaard et al., 1996).
Diabetes mellitus is marked by the inability of pancreatic β-cells to produce any or enough insulin, resulting in a myriad of health issues like blindness, renal failure and nerve damage; the risk of heart disease, stroke and disease of the blood vessels increases over time as well (Amin et al., 2006). According to the American Diabetes Association, 20.8 million children and adults in the United States currently have diabetes, with another 54 million being classified as pre-diabetic. The total healthcare costs of diabetes in the United States exceed $130 billion annually (2005). There are two major types of diabetes – Type 1 and Type 2 – as well as gestational diabetes, a condition that affects women during pregnancy.
Type 1 diabetes is an auto-immune disease that is a result of lesions on the β-cells that leads to insulin deficiency. It often occurs in the young but can appear at any age (Kuzuya et al., 2002). Approximately 70–80% of the β-cells have been destroyed by the time Type 1 diabetes is diagnosed. Because of the absence of the body's functionality to produce insulin to regulate glucose levels, supplemental insulin and constant monitoring are required to maintain normal function (Cnop et al., 2005).
Type 2 diabetes is the more prevalent of the two types of diabetes, accounting for 90–95% of the diagnosed cases (2005). A progressive increase in insulin resistance results in the inability of the pancreas to produce the hormone. This often occurs in the older portion of the population and can be associated with obesity and genetics, among other factors (Ross et al., 2004). As the inability to produce insulin escalates, supplemental insulin is needed to maintain the necessary levels. Gestational diabetes occurs in pregnant women when treatment is needed to stabilize blood glucose levels in order to maintain a healthy environment for the fetus.
Normoglycemia is achieved in the blood glucose range of 70–120 mg/dL (Amin et al., 2006). Blood glucose levels must be maintained at two states: mealtimes and between meals. To counteract the glucose introduced at a meal, short-acting insulin is injected prior to the meal so that postprandial glucose control can be achieved. For the times between meals, a basal level of insulin must be maintained.
Insulin therapy is primarily via injection, but the variety of injection methods is more diverse today than ever. In addition to traditional syringe injections, pen devices and insulin pumps exist on the market that eliminate some of the associated pain with injection therapy and provide more accurate dosage control over manually filled syringes (Heller et al., 2007).
Inhalable insulin arrived on the market with Exubera®, the product of a three-way collaboration between Sanofi-Aventis, Nektar Therapeutics and Pfizer. Exubera® demonstrated similar pharmacokinetic behavior as injected short-acting insulin (Amin et al., 2008), but poor marketing, unappealing design and a lack of physician support led to the product being abandoned in October 2007 (Opar, 2008).
The intranasal delivery of insulin has been attempted by many researchers to mixed results. In human studies, relative bioavailabilities are often less than 20% with high degrees of variability (Owens et al., 2003). Intranasal delivery of an insulin-chitosan powder achieved a relative bioavailability of 17.0 ± 6.6% as opposed to a pure intranasal insulin solution bioavailability of 0.5 ± 0.1% (Dyer et al., 2002). The chitosan acted as a permeation enhancer to enhance the bioavailability in this formulation. A novel device was tested by Pringels et al. for the delivery of an insulin/starch/Carbopol® 974P powder. These studies demonstrated an absolute bioavailability of 14.4 ± 3.5% for the experimental device (Pringels et al., 2006).
An insulin buccal spray developed with soybean lecithin and propanediol resulted in a relative bioavailability of 29.2 ± 11.1% in rabbits with a hypoglycemic effect lasting more than 4h (Xu et al., 2002). Mucoadhesive chitosan sponges have also shown promising in vitro release data but have not been tested in an animal model to date (Portero et al., 2007). Generex Biotechnology has developed Oral-lyn™ for the treatment of Type 1 and Type 2 diabetes; it is currently on sale in Ecuador, approved for sale in India, and is in Phase III clinical trials in the United States and other countries.
Growth Hormone
Growth hormone, or somatotropin, is a single polypeptide chain consisting of 191 amino acids. The molecular weight varies based on the species of origin: human, 22124 Da; bovine, 22256 Da. The isoelectric point of growth hormone is 4.9 and the chain has a hydrodynamic radius of approximately 24–25 Å (Rabkin et al., 1981; Shamlou et al., 2007). The protein is synthesized in and released from somatotrophs (Harvey, 1995), a cell type located in the anterior pituitary gland at the base of the brain. Release of growth hormone is regulated by growth hormone release hormone (GHRH) and somatostatin (SRIF). GHRH is responsible for the release of growth hormone by the induction of extracellular calcium entry into the somatotrophs. SRIF acts to block the cellular uptake of calcium, resulting in no release from the somatotrophs. As SRIF only limits release of the protein and not synthesis, more drug is initially released upon GHRH action due to pooling effects within the production site (Harvey, 1995).
Growth hormone deficiency is an abnormality occurring in 20,000 children and approximately 50,000 adults in the United States (Gharib, 2003). It is marked by a lack of secretion by the pituitary gland, resulting in hyposomatotropism. One possible reason for this is a hypothalamic problem leading to insufficient or abnormal synthesis of GHRH. The result of growth hormone deficiency is a decelerated growth rate or growth failure (Daughaday and Harvey, 1995). Furthermore, in adults, certain cardiovascular risk factors are heightened, including an increase in fat content and insulin resistance (Gharib, 2003). The deficiency can be treated by supplementary administration of recombinant human growth hormone (rhGH).
Additional conditions that can be treated with growth hormone therapy include Turner syndrome and Prader-Willi syndrome. Turner syndrome occurs in females when all or part of an X chromosome is lost, resulting in estrogen deficiency and short stature among a myriad of other markers (Landin-Wilhelmsen et al., 2001). Prader-Willi syndrome is characterized by obesity, short stature and mental retardation, and growth hormone therapy aids in improving body composition and lean body mass (Carrel et al., 2004). Chronic renal failure and muscular wasting in advanced AIDS patients (Gharib, 2003) are also treated by growth hormone therapy.
The primary administration route of rhGH is injection. The recommended dosing levels vary for adults and children, but daily dosings are required in most cases (Gharib, 2003). While some rhGH formulations are available in pen delivery devices that eliminate some of the pain of injection, the daily dosing necessary for most growth hormone dependent patients amplifies the need for an improved administration route.
The pulmonary delivery of growth hormone has been investigated and found to have mixed results depending on the method of delivery. Studies of pulmonary delivery in rats and rabbits indicated bioavailabilities between 5% and 45% and the bioavailability in humans has been reported at 5–10%. An absolute bioavailability of 23% via pulmonary delivery relative to a subcutaneous bioavailability of 56% has been reported in vivo (Bosquillon et al., 2004). The dry powder aerosol used in the aforementioned study had a mass median aerodynamic diameter of 4.4 μm, small enough for alveolar absorption.
Researchers have investigated the delivery of recombinant human growth hormone in a sheep model. Relative to a subcutaneous injection of rhGH at a dose of 0.03 mg/kg, the bioavailabilities achieved in formulations with chitosan at a dose of 0.3 mg/kg were 14 ± 9% (powder blend) and 15 ± 8% (granules) (Cheng et al., 2005). Further examples of intranasal delivery of growth hormone may be found in a later section on thiolated polymers (Leitner et al., 2004). No evidence of a buccal formulation for growth hormone could be found in the literature. The size of the protein (22 kDa) may limit the feasibility of utilizing the buccal cavity for this drug.
Calcitonin
Calcitonin is a 32 amino acid single chain peptide hormone produced in the parafollicular cells, or C cells, of the thyroid gland (Copp et al., 1962). Calcitonin has a molecular weight of 3432 Da (Cui and Mumper, 2002) and is marked by a high isoelectric point of 8.86 (Torres-Lugo and Peppas, 1999). Three classes of calcitonin have been identified and each has a different level of potency to inhibit bone resorption. In order of highest to lowest potency, they are teleost/avian, artiodactyl, and primate. The non-mammalian calcitonins have more biological potency because of their more basic structure. It is for this reason that salmon calcitonin, part of the teleost/avian classification, is often used in mammals (Pondel, 2000).
Osteoporosis and Paget's disease are two of the most prevalent diseases for which calcitonin is used as a treatment. It is also used in the treatment of hypercalcaemia and bone metastases. Osteoporosis is marked by low bone density and deterioration of the tissue within the bone, resulting in increasing fragility (Pinkerton and Dalkin, 2007). An estimated 28 million women in the United States have either osteoporosis or are at risk to be diagnosed with the disease due to low bone mass (Wehren, 2002). Calcitonin is used in osteoporotic patients to prevent bone loss and increase bone mineral density (BMD). It has been shown that the drug is also able to reduce the risk of fracture as BMD decreases (Ellerington et al., 1996) and provide an analgesic, pain-relieving effect (Azria, 2002; Lyritis et al., 1999).
Paget's disease is marked by an abnormal increase in bone resorption. It is the second most common skeletal disorder following osteoporosis and affects 2–5% of the population older than 50. The disorder leads to an increase in bone formation and destroys normal bone architecture (Papapoulos, 1997). Recent studies suggest that the prevalence of the disease is decreasing worldwide (Poór et al., 2006) but treatment options are still necessary for those afflicted. Calcitonin has been shown to reduce the rate of bone turnover by 50%, but the eventual development of a resistance to the drug makes long-term management of the disease difficult (Papapoulos, 1997). Papapoulos comments that the need for frequent injections is one cause for the difficulty in managing this disease chronically.
The primary administration route of calcitonin is via injection, however nasal (Overgaard et al., 1992; Overgaard et al., 1989), buccal (Cui and Mumper, 2002) and transdermal delivery methods have been reported. Commercially, subcutaneous or intramuscular injections and nasal formulations are available. Oral formulations have been investigated (Lee and Sinko, 2000) and an oral preparation is under clinical development at this time (Chesnut et al., 2008).
Gelatin microspheres have been used for the pulmonary delivery of salmon calcitonin. As the size of the microspheres dropped from 71.2 μm to 3.4 μm, the pharmacological availability of salmon calcitonin delivered via positively charged gelatin increased from 24.7 ± 3.8% to 51.3 ± 10.3%. Negatively charged microspheres at 10.9 μm yielded an availability of 35.4 ± 6.5% (Morimoto et al., 2000).
Nasal delivery of salmon calcitonin has been met with mixed results. One study, testing the effectiveness of a nasal spray for the treatment of lumbar canal stenosis, found that there were no benefits of administering calcitonin nasally as opposed to placebo (Podichetty et al., 2004). However, formulations of nasal calcitonin with alkylglycosides have been able to achieve relative bioavailabilities of 53% and rapid absorption (Ahsan et al., 2001). These mixed results – both with the commercially available form of calcitonin known as Miacalcin® (Novartis Pharmaceuticals Corp.) – demonstrate the high degree of variability with the nasal route that needs to be controlled in order to consider this route a viable option for protein delivery.
Bilayer thin film composites of Eudragit® S-100, polycarbophil, and utility wax have been tested for the buccal delivery of salmon calcitonin. Minimum plasma calcium concentration levels were seen after 120–150 min for both the intravenous and buccal routes. The relative bioavailability for the buccal calcitonin in a rabbit model was 43.8 ± 10.9% (Cui and Mumper, 2002)
SYSTEMS FOR THE ORAL DELIVERY OF PROTEINS
Proteins delivered by the oral route must be protected from the harsh conditions faced in the gastrointestinal tract in order to maintain their pharmacological activity. One option to achieve this – protease inhibitors in the formulation – has the potential to dangerously alter the enzymatic functions mandated by the body's dietary needs. Additionally, the intestinal barrier limits the amount of protein that can be absorbed. An alternative to using chemical means to protect the protein is to use a carrier that protects the drug from degradation. This option may be used for site-specific drug delivery as well, but proper design measures must be implemented to allow for drug release at the desired location.
Enteric Coatings
Enteric coatings can be used to provide site-specific delivery at different regions of the gastrointestinal tract. The coatings are polymer layers that dissolve upon reaching a specific pH value and thus can be used to encapsulate tablets or capsules from the conditions found in the stomach. Perhaps the most popular brand of enteric coatings is Eudragit®, a product of Degussa, Germany. The Eudragit® coatings for small intestinal delivery (L100, L30 and S100) are based on methacrylic acid and copolymers with materials like methyl methacrylate. The polymers are not crosslinked and thus can dissolve readily upon reaching precise pH values (5.5 for the duodenum and 6.0 for the jejunum). The coatings do not have any effect on improving intestinal permeability, though, and strictly provide protection from degradation.
Thiolated Polymers
Thiolated polymers, or thiomers, are a class of materials that have shown particular promise as drug delivery carriers for their mucoadhesive properties. Thiomers, in opposition to systems that are capable of forming hydrogen bonds and ionic interactions with the gastrointestinal mucosa, are capable of forming covalent bonds in the form of disulfide linkages. These bonds are with cysteine-rich regions of the mucus glycoproteins (Bernkop-Schnürch, 2005; Leitner et al., 2003). Amide and amidine bonds, when facilitated by chemistries like carbodiimide chemistry, can be used to immobilize thiol groups to cationic and anionic polymers to form thiomers (Bernkop-Schnürch et al., 1999; Bernkop-Schnurch et al., 2001; Kast and Bernkop-Schnurch, 2001). The number of immobilized thiol groups directly correlates to the enhancement of the mucoadhesive properties of the material (Bernkop-Schnürch, 2005). In addition to their mucoadhesive properties, thiomers are noted for their permeation enhancement and enzyme inhibitory capabilities (Bernkop-Schnurch et al., 2004).
Thiomers have been incorporated with transmucosal delivery formulations for various drugs, including heparin (Kast et al., 2003), insulin (Calceti et al., 2004; Marschutz et al., 2000), human growth hormone (Leitner et al., 2004; Leitner et al., 2004), and salmon calcitonin (Guggi et al., 2003). The routes for these deliveries have included the buccal, nasal, ocular, oral and vaginal mucosas (Werle, 2008). Oral delivery of PEGylated insulin with the thiolated polymer poly(acrylic acid)-cysteine yielded a bioavailability of 7% in diabetic mice (Calceti et al., 2004). Polycarbophil-cysteine conjugates were incorporated in an enteric coated tablet with insulin and dropped the percentage of degraded insulin from 90% in an unprotected state to 50% in the protected state (Marschutz et al., 2000). Bioavailability of human growth hormone via the nasal route was 2.75% when the protein was administered with the permeation enhancer polycarbophil cysteine/glutathione (Leitner et al., 2004) as a gel, but this value was increased to 8.11 ± 2.15% when the protein was administered as a microparticulate formulation (Leitner et al., 2004). In vivo decreases in the plasma calcium level were found to be 10% when salmon calcitonin was delivered with a chitosan-4-thiobutylamidine enzyme inhibiting conjugate in comparison to a 20.5 ± 5.3% decrease when the drug was administered intravenously.
The primary difference between thiomers being utilized as enzyme inhibitors or permeation enhancers is that their effects are localized to where the mucoadhesion occurs, which might result in less toxic effects than those expected with a non site-specific administered entity (Werle and Bernkop-Schnurch, 2006). It is possible, though, that the enhancement of intestinal residence time to upwards of 10 h in the presence of these permeation enhancing thiomers could allow for the uptake of pathogens that the intestinal mucosa is designed to protect against.
Stimuli-Sensitive Hydrogels
Hydrogels are hydrophilic materials that possess many properties that lend themselves for use in drug delivery systems, including their biocompatibility, swelling potential, and ability for some of the gels to respond to stimuli like temperature, pH, and analytes (Hoffman, 2001; Langer and Peppas, 2003). The physical integrity of these networks is provided by chemical or physical crosslinks, such as entanglements or crystallites (Peppas et al., 2000). Characteristics, such as their resemblance to living tissue and biocompatibility, make them suitable for numerous medical and pharmaceutical applications. Examples of these applications include: contact lenses, membranes for biosensors, linings for artificial hearts, materials for artificial organs and drug delivery devices (Peppas and Langer, 1994).
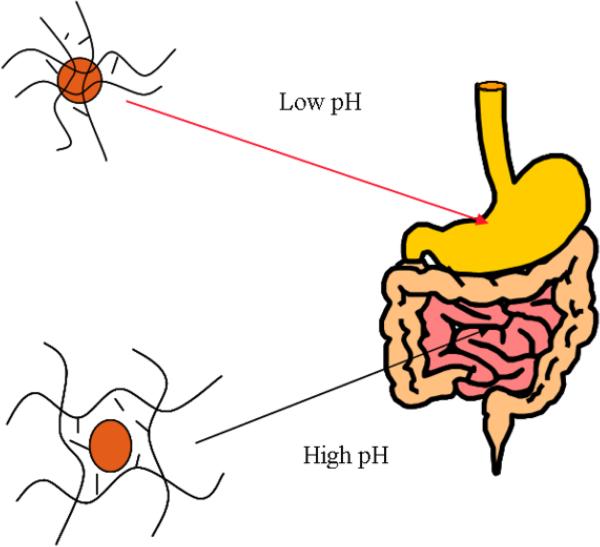
Stimuli-sensitive hydrogels are a class of carriers that have been rigorously evaluated for site-specific drug delivery (Peppas et al., 2000). With the oral delivery of proteins, the site of interest is the upper small intestine and the dominant stimulus in the transition between the stomach and small intestine is pH. pH-sensitive gels, therefore, have been investigated for use in oral delivery applications. These gels can be neutral, ampholytic, cationic or anionic (Peppas, 2004). Cationic gels are swollen at a pH below the pKa of the ionic constituent and deswell above the pKa; anionic gels operate in the reverse manner and are swollen at conditions above the pKa. Since the pH transition in the GI tract is from low to high, anionic gels provide the best option for a pH-sensitive hydrogel carrier for the oral delivery of proteins. At low pH values, the polymer network is in its complexed state allowing the necessary protection of the drug from the harsh and acidic environment. Then, in the neutral environment of the small intestine, at pH 6 to 7, the polymer network is in its swollen state, allowing release of the drug (See Figure 6).
Figure 6.
Anionic complexation hydrogel swelling behavior as a function of the pH of the gastrointestinal tract.
Hydrogels are chemically crosslinked to form a three-dimensional matrix that is capable of imbibing large amounts of water or biological fluids (Peppas and Mikos, 1986). The matrix provides a location for drugs to be loaded and shielded until the appropriate time of release. In addition to the chemical crosslinks found in hydrogel systems, physical crosslinks in the form of complexes can form between constituents in copolymer or multipolymer systems. When using polyacids like poly(methacrylic acid) or poly(acrylic acid), these complexes take the form of hydrogen bonding complexes between polyacid and nonionic constituents (Bekturov and Bimendina, 1981). Interpolymer complexes that are stabilized by hydrogen bonds form between electron deficient groups like polyacids, and electron dense groups like ethers and pyrrolidones (Lowman and Peppas, 2000).
Swelling for these gels is controlled by thermodynamic compatibility of the polymer and the swelling medium (Lowman and Peppas, 1999). Swelling would continue until infinite dilution was reached if physical and chemical crosslinks were not present in the network. The crosslinks oppose additional swelling and a point of equilibrium swelling is reached (Kashyap et al., 2005). The period leading up to this moment can be considered to be a state of dynamic swelling.
P(MAA-g-PEG)
One of the most extensively investigated hydrogel systems for the oral delivery of proteins is a copolymer of methacrylic acid with grafted poly(ethylene glycol) chains, designated as P(MAA-g-PEG) (Lopez and Peppas, 2004; Morishita et al., 2006). The system is an anionic hydrogel and is swollen in intestinal conditions but remains collapsed in the gastric environment. Poly(ethylene glycol) is incorporated for both its high level of biocompatibility and its ability to form hydrogen bonding complexes with methacrylic acid (Kim and Peppas, 2003). The protons of the carboxylic acid groups on PMAA form hydrogen bonds with ether groups on the PEG chain (Bell and Peppas, 1996). Poly(ethylene glycol) (PEG) has been widely used in medical and pharmaceutical applications because of its biocompatibility. In addition to being non-toxic, the PEG tethered chains act as bioadhesion promoters on the surface of the polymer network. The mucoadhesive properties have led to improved retention times in the small intestine (Goto et al., 2006).
The environmental responsive ability of P(MAA-g-PEG) was first investigated by Klier et al. (1990). Peppas and Klier (1991) also studied the potential of this polymer network for a drug delivery system. Other properties, such as the length of the grafted PEG chains, were further investigated by Bell and Peppas (1996). They also showed that PEG chains with molecular weight of 1000 Da had the highest degree of complexation in low pH environments. The largest amounts of complexation were also obtained when equimolar amounts of the monomeric MAA and PEG were used in the synthesis of the polymeric network (Lowman and Peppas, 2000).
The network correlation length, or mesh size, of these complexes is the end-to-end distance of the swollen polymer chains between junction points. Lowman and Peppas calculated this parameter for complexed and swollen P(MAA-g-PEG) polymer networks resulting in values of 70 Å and 210 Å, respectively (Lowman and Peppas, 1997). This desired swelling behavior lead to the investigation of this system for the oral delivery of proteins such as insulin (Ichikawa and Peppas, 2001; Lowman et al., 1999) and calcitonin (Torres-Lugo and Peppas, 1999).
Madsen and Peppas (1999) investigated the ability of the P(MAA-g-PEG) network to bind divalent cations such as Ca2+ resulting in inhibition of calcium dependent proteolytic enzymes such as trypsin. This behavior was attributed to the presence of negatively charged carboxylic acid in the polymer network. The presence of the microparticles has been shown to prevent cell and tissue damage for extended periods of time (Sipahigil et al., 2006). The similar system of acrylic acid and poly(ethylene glycol), or P(AA-g-PEG), has been shown to demonstrate some of these beneficial properties as well (Foss et al., 2004; Serra et al., 2006; Thomas et al., 2007).
Cell studies carried out by Torres-Lugo et al. (Torres-Lugo et al., 2002) and Foss and Peppas (Foss and Peppas, In Press) showed that P(MAA-g-EG) microparticles and nanospheres were not toxic when they were in contact with the Caco-2 epithelial cell monolayer. They also showed that they were able to decrease the transepithelial electrical resistance (TEER) of the monolayer, favoring the transport of the protein through the paracellular route because of the disruption of the tight junctions. Moreover, this behavior was found to be reversible. Finally, the transport of insulin through the cell monolayer in the presence of the P(MAA-g-EG) microparticles and nanospheres has been investigated (Foss and Peppas, In Press; Torres-Lugo et al., 2002).
Microparticles of the P(MAA-g-PEG) system have been shown to have high insulin incorporation efficiencies and in vivo bioavailabilities of 4.22% in 50 IU/kg doses. Comparatively, a 25 IU/kg dose in P(MAA-g-PEG) microparticles revealed a bioavailability of 3.4% whereas Eudragit L100 enteric coatings only had a bioavailability of 0.9%. Lowman et al. (1999) performed animal studies and showed that following the administration of insulin loaded P(MAA-g-EG) microparticles to healthy and diabetic rats, the blood glucose levels in these animals decreased significantly for at least 8 h due to the absorption of insulin in the gastrointestinal tract.
Recent optimizations on this system – particularly in the size of microparticle tested – have led to an increase in the relative bioavailability of insulin to 12.8 ± 8.3% in a murine model with microparticles smaller than 43 μm in diameter (Morishita et al., 2004). Furthermore, the system has been successfully utilized in the delivery of salmon calcitonin (Torres-Lugo et al., 2002; Torres-Lugo and Peppas, 1999), insulin-transferrin conjugates (Kavimandan et al., 2006), and other drugs like chemotherapeutic agents (Blanchette and Peppas, 2005; Blanchette and Peppas, 2005).
The P(MAA-g-PEG) system is a very promising candidate for the oral delivery of proteins. It could be described as a compilation of all the different approaches being investigated for the delivery of proteins via the oral route. This polymer network shows a desired pH-dependent behavior, protecting the protein from the harsh environment in the stomach and releasing it in the target area of the small intestine. It also is an inhibitor and complexing agent because of its ability to bind divalent cations and inhibit proteolytic enzymes. And finally, the PEG tethered chains give the polymer the desired mucoadhesive properties.
There are limitations to the P(MAA-g-PEG) system that could be improved upon in order to develop an optimal system for oral protein delivery. The presence of PEG in the hydrogel has been shown to delay protein release upon reaching intestinal pH levels (Nakamura et al., 2004) and P(MAA-g-PEG) does not provide full protection in gastric pH levels where drug release should be inhibited (Besheer et al., 2006; Wood et al., 2008). Furthermore, Moriyama et al. have reported that insulin partitioned into a PEG phase at physiological pH levels in the presence of a negatively charged polyelectrolyte (Moriyama et al., 1999). Although there have been a great number of successes reported with the P(MAA-g-PEG) hydrogel system and others in the literature, these limitations demonstrate that it is imperative to continue the search for materials that complex more effectively, provide better protection from enzymatic degradation and premature release, and improve pharmacological availability so that the development of an optimal carrier for the oral delivery of therapeutic proteins may be achieved.
CONCLUSIONS
Scientific advances have led to the development of new biomaterials that can successfully deliver therapeutic proteins to the bloodstream via several physiological routes. The apparent simplicity of delivering a compound via one of these routes is overshadowed by the biological conditions that limit the achievement of sufficient absorption and transport of the therapeutic agent. The oral route is a perfect example of this dichotomy. It is both patient friendly and cost-effective, but the complex nature of the organs and conditions in the gastrointestinal tract mandates a robust investigation when developing novel materials for protein delivery.
Utilizing cell culture and other laboratory techniques, a significant understanding of the physics of the problem has been achieved in the last few years. A number of successful protein delivery formulations like thiomers and stimuli-sensitive complexation hydrogels have been designed, tested and proven to be improvements over the work of earlier generations. However, as bioavailabilities for many of the successful formulations of today remain low in comparison to injection, a fundamental transport analysis of protein delivery remains an important task. The development of models that could accurately predict the protein blood concentration as a function of the structure and characteristics of protein delivery systems would greatly enhance the capabilities of researchers, potentially ushering in a new class of materials that provide better absorption and transport of protein therapeutic agents.
ACKNOWLEDGMENTS
This work was supported by grant No. R01 EB-00246-16 from the National Institutes of Health, and a National Science Foundation Graduate Research Fellowship to DAC.
Footnotes
This article is dedicated to Professor Morton M. Denn on the occasion of his 70th birthday. The senior author (NAP) has had a 35-year admiration for Denn's scholarship and deep understanding of the field of transport phenomena. In his early days he was very much affected by Professor Denn's relentless pursuit of high quality, scholarship and excellence. He has learned from Dr. Denn's pioneering publications and books on polymer rheology and fluid mechanisms. Indeed, his modeling of protein transport through the intestinal wall has been affected by Professor Denn's pioneering work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agu RU, Ugwoke MI, Armand M, Kinget R, Verbeke N. The lung as a route for systemic delivery of therapeutic proteins and peptides. Respiratory Research. 2001;2(4):198–209. doi: 10.1186/rr58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahsan F, Arnold J, Meezan E, Pillion DJ. Enhanced bioavailability of calcitonin formulated with alkylglycosides following nasal and ocular administration in rats. Pharmaceutical Research. 2001;18(12):1742–1746. doi: 10.1023/a:1013330815253. [DOI] [PubMed] [Google Scholar]
- Amin A, Shah T, Patel J, Gajjar A. Non-invasive Insulin Delivery Technologies: Current Trends & Future Prospects. Drug Delivery Technology. 2006;6(3):48–60. [Google Scholar]
- Amin A, Shah T, Patel J, Gajjar A. Non-invasive Insulin. Drug Delivery Technology. 2008;8(3):43–48. [Google Scholar]
- Arora P, Sharma S, Garg S. Permeability issues in nasal drug delivery. Drug Discovery Today. 2002;7(18):967–975. doi: 10.1016/s1359-6446(02)02452-2. [DOI] [PubMed] [Google Scholar]
- Artursson P. Epithelial Transport of Drugs I. A Model for Studying the Transport of Drugs (β-blocking agents) Over an Intestinal Epithelial Cell Line (Caco-2) Journal of Pharmaceutical Science. 1990;79:1123–1129. doi: 10.1002/jps.2600790604. [DOI] [PubMed] [Google Scholar]
- Artursson P, Palm K, Luthman K. Caco-2 Monolayers in Experimental and Theoretical Predictions of Drug Transport. Advanced Drug Delivery Reviews. 2001;46:27–43. doi: 10.1016/s0169-409x(00)00128-9. [DOI] [PubMed] [Google Scholar]
- Audus KL, Bartel RL, Hidalgo IJ, Borchardt RT. The Use of Cultured Epithelial and Endothelial Cells for Drug Transport and Metabolism Studies. Pharmaceutical Research. 1990;7:435–451. doi: 10.1023/a:1015800312910. [DOI] [PubMed] [Google Scholar]
- Aungst BJ. Intestinal permeation enhancers. Journal of Pharmaceutical Sciences. 2000;89(4):429–442. doi: 10.1002/(SICI)1520-6017(200004)89:4<429::AID-JPS1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Azria M. Possible mechanisms of the analgesic action of calcitonin. Bone. 2002;30(5):80S–83S. doi: 10.1016/s8756-3282(02)00701-9. [DOI] [PubMed] [Google Scholar]
- Bajpai A, Menon PSN. Growth hormone therapy. Indian Journal of Pediatrics. 2005;72(2):139–144. doi: 10.1007/BF02760699. [DOI] [PubMed] [Google Scholar]
- Bekturov EA, Bimendina LA. Interpolymer complexes. Advances in Polymer Science. 1981;43:100–147. [Google Scholar]
- Bell CL, Peppas NA. Swelling/Syneresis Phenomena in Gel-Forming Interpolymer Complexes. Journal of Biomaterials Science, Polymer Edition. 1996;7:671–683. doi: 10.1163/156856296x00444. [DOI] [PubMed] [Google Scholar]
- Bell CL, Peppas NA. Modulation of Drug Permeation Through Interpolymer Complexed Hydrogels for Drug Delivery Applications. Journal of Controlled Release. 1996;39:201–207. [Google Scholar]
- Bernkop-Schnurch A. The use of inhibitory agents to overcome the enzymatic barrier to perorally administered therapeutic peptides and proteins. Journal of Controlled Release. 1998;52(1–2):1–16. doi: 10.1016/s0168-3659(97)00204-6. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnürch A. Thiomers: A new generation of mucoadhesive polymers. Advanced Drug Delivery Reviews. 2005;57(11):1569–1582. doi: 10.1016/j.addr.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnürch A, Brandt UM, Clausen AE. Synthesis and in vitro evaluation of chitosan-cysteine conjugates. Sci Pharm. 1999;67:196–208. [Google Scholar]
- Bernkop-Schnurch A, Hoffer MH, Kafedjiiski K. Thiomers for oral delivery of hydrophilic macromolecular drugs. Expert Opin Drug Deliv. 2004;1(1):87–98. doi: 10.1517/17425247.1.1.87. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnurch A, Kast CE, Richter MF. Improvement in the mucoadhesive properties of alginate by the covalent attachment of cysteine. Journal of Controlled Release. 2001;71(3):277–285. doi: 10.1016/s0168-3659(01)00227-9. [DOI] [PubMed] [Google Scholar]
- Besheer A, Wood KM, Peppas NA, Mader K. Loading and mobility of spin-labeled insulin in physiologically responsive complexation hydrogels intended for oral administration. Journal of Controlled Release. 2006;111(1–2):73–80. doi: 10.1016/j.jconrel.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Blanchette J, Kavimandan N, Peppas NA. Principles of transmucosal delivery of therapeutic agents. Biomedicine and Pharmacotherapy. 2004;58(3):142–151. doi: 10.1016/j.biopha.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Blanchette J, Peppas NA. Cellular evaluation of oral chemotherapy carriers. Journal of Biomedical Materials Research, Part A. 2005;72A(4):381–388. doi: 10.1002/jbm.a.30243. [DOI] [PubMed] [Google Scholar]
- Blanchette J, Peppas NA. Oral chemotherapeutic delivery: Design and cellular response. Annals of Biomedical Engineering. 2005;33(2):142–149. doi: 10.1007/s10439-005-8973-8. [DOI] [PubMed] [Google Scholar]
- Bosquillon C, Préat V, Vanbever R. Pulmonary delivery of growth hormone using dry powders and visualization of its local fate in rats. Journal of Controlled Release. 2004;96(2):233–244. doi: 10.1016/j.jconrel.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Braun A, Hämmerle S, Suda K, Rothen-Rutishauser B, Günthert M, Krämer SD, Wunderli-Allenspach H. Cell Cultures as Tools in Biopharmacy. European Journal of Pharmaceutical Science. 2000;11:S51–S60. doi: 10.1016/s0928-0987(00)00164-0. [DOI] [PubMed] [Google Scholar]
- Brønsted H, Kopeček J. Hydrogels for Site-Specific Drug Delivery to the Colon: In Vitro and In Vivo Degradation. Pharmaceutical Research. 1992;9:1540–1545. doi: 10.1023/a:1015847921435. [DOI] [PubMed] [Google Scholar]
- Calceti P, Salmaso S, Walker G, Bernkop-Schnürch A. Development and in vivo evaluation of an oral insulin-PEG delivery system. European Journal of Pharmaceutical Sciences. 2004;22(4):315–323. doi: 10.1016/j.ejps.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Carino GP, Mathiowitz E. Oral insulin delivery. Advanced Drug Delivery Reviews. 1999;35(2–3):249–257. doi: 10.1016/s0169-409x(98)00075-1. [DOI] [PubMed] [Google Scholar]
- Carrel AL, Moerchen V, Myers SE, Bekx MT, Whitman BY, Allen DB. Growth hormone improves mobility and body composition in infants and toddlers with Prader-Willi syndrome. Journal of Pediatrics. 2004;145(6):744–749. doi: 10.1016/j.jpeds.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Cheng Y-H, Dyer AM, Jabbal-Gill I, Hinchcliffe M, Nankervis R, Smith A, Watts P. Intranasal delivery of recombinant human growth hormone (somatropin) in sheep using chitosan-based powder formulations. European Journal of Pharmaceutical Sciences. 2005;26(1):9–15. doi: 10.1016/j.ejps.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Chesnut CH, Azria M, Silverman S, Engelhardt M, Olson M, Mindeholm L. Salmon calcitonin: a review of current and future therapeutic indications. Osteoporosis International. 2008;19(4):479–491. doi: 10.1007/s00198-007-0490-1. [DOI] [PubMed] [Google Scholar]
- Chickering DE, Mathiowitz E. Definitions, Mechanisms, and Theories of Bioadhesion. In: Mathiowitz E, Chickering DE, Lehr C-M, editors. Bioadhesive drug delivery systems: fundamentals, novel approaches, and development. Marcel Dekker; New York: 1999. [Google Scholar]
- Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes - Many differences, few similarities. Diabetes. 2005;54:S97–S107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- Cogburn JN, Donovan MG, Schasteen CS. A Model of Human Small Intestinal Absorptive Cells. 1. Transport Barrier. Pharmaceutical Research. 1991;8:210–216. doi: 10.1023/a:1015844104539. [DOI] [PubMed] [Google Scholar]
- Copp DH, Davidson AG, Henze KG, Cheney BA, Cameron EC. Evidence for Calcitonin - a New Hormone from Parathyroid That Lowers Blood Calcium. Endocrinology. 1962;70(5):638–649. doi: 10.1210/endo-70-5-638. [DOI] [PubMed] [Google Scholar]
- Cui ZR, Mumper RJ. Buccal transmucosal delivery of calcitonin in rabbits using thin-film composites. Pharmaceutical Research. 2002;19(12):1901–1906. doi: 10.1023/a:1021462012442. [DOI] [PubMed] [Google Scholar]
- Daughaday WH, Harvey S. Growth Hormone Release: Pathophysiological Dysfunction. In: Harvey S, Scanes CG, Daughaday WH, editors. Growth Hormone. CRC Press; Boca Raton: 1995. [Google Scholar]
- Dodou D, Breedveld P, Wieringa PA. Mucoadhesives in the gastrointestinal tract: revisiting the literature for novel applications. European Journal of Pharmaceutics and Biopharmaceutics. 2005;60(1):1–16. doi: 10.1016/j.ejpb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Dyer AM, Hinchcliffe M, Watts P, Castile J, Jabbal-Gill I, Nankervis R, Smith A, Illum L. Nasal delivery of insulin using novel chitosan based formulations: A comparative study in two animal models between simple chitosan formulations and chitosan nanoparticles. Pharmaceutical Research. 2002;19(7):998–1008. doi: 10.1023/a:1016418523014. [DOI] [PubMed] [Google Scholar]
- Ellerington MC, Hillard TC, Whitcroft SIJ, Marsh MS, Lees B, Banks LM, Whitehead MI, Stevenson JC. Intranasal salmon calcitonin for the prevention and treatment of postmenopausal osteoporosis. Calcified Tissue International. 1996;59(1):6–11. doi: 10.1007/s002239900076. [DOI] [PubMed] [Google Scholar]
- Espinal J. Understanding insulin action : principles and molecular mechanisms. Ellis Horwood Limited; Chichester, England: [Google Scholar]
- Fasano A, Uzzau S. Modulation of Intestinal Tight Junctions by Zonula Occludens Toxin Permits Enteral Administration of Insulin and Other Macromolecules in an Animal Model. Journal of Clinical Investigation. 1997;99:1158–1164. doi: 10.1172/JCI119271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A. Innovative strategies for the oral delivery of drugs and peptides. Trends in Biotechnology. 1998;16(4):152–157. doi: 10.1016/s0167-7799(97)01170-0. [DOI] [PubMed] [Google Scholar]
- Fix JA. Oral Controlled Release Technology for Peptides: Status and Future Prospects. Pharmaceutical Research. 1996;13:1760–1764. doi: 10.1023/a:1016008419367. [DOI] [PubMed] [Google Scholar]
- Foss A, Peppas NA. The effect of Acrylic-Based Copolymers on Caco-2 Cultures. Journal of Biomedical Materials Research. In Press. [Google Scholar]
- Foss AC, Goto T, Morishita M, Peppas NA. Development of acrylic-based copolymers for oral insulin delivery. European Journal of Pharmaceutics and Biopharmaceutics. 2004;57(2):163–169. doi: 10.1016/S0939-6411(03)00145-0. [DOI] [PubMed] [Google Scholar]
- Gan LL, Yanni S, Thakker DR. Modulation of the Tight Junctions of the Caco-2 Cell Monolayer by H2-antagonist. Pharmaceutical Research. 1998;15:53–57. doi: 10.1023/a:1011944602662. [DOI] [PubMed] [Google Scholar]
- Generex Biotechnology. [cited 2 May 2008]; Available from: http://www.generex.com/index.php.
- Gerich JE. Novel insulins: expanding options in diabetes management. American Journal of Medicine. 2002;113(4):308–316. doi: 10.1016/s0002-9343(02)01176-2. [DOI] [PubMed] [Google Scholar]
- Gharib H. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for Growth Hormone Use in Adults and Children - 2003 Update. Endocrine Practice. 2003;9(1):64–76. doi: 10.4158/EP.9.1.64. [DOI] [PubMed] [Google Scholar]
- Goto T, Morishita M, Kavimandan NJ, Takayama K, Peppas NA. Gastrointestinal transit and mucoadhesive characteristics of complexation hydrogels in rats. Journal of Pharmaceutical Sciences. 2006;95(2):462–469. doi: 10.1002/jps.20566. [DOI] [PubMed] [Google Scholar]
- Grabovac V, Guggi D, Bernkop-Schnürch A. Comparison of the mucoadhesive properties of various polymers. Advanced Drug Delivery Reviews. 2005;57(11):1713–1723. doi: 10.1016/j.addr.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Granger DN, Barrowman JA, Kvietys PR. Clinical Gastrointestinal Physiology. W.B. Saunders Company; Philadelphia: [Google Scholar]
- Guggi D, Kast CE, Bernkop-Schnurch A. In vivo evaluation of an oral salmon calcitonin-delivery system based on a thiolated chitosan carrier matrix. Pharmaceutical Research. 2003;20(12):1989–1994. doi: 10.1023/b:pham.0000008047.82334.7d. [DOI] [PubMed] [Google Scholar]
- Hamman JH, Enslin GM, Kotze AF. Oral delivery of peptide drugs - Barriers and developments. BioDrugs. 2005;19(3):165–177. doi: 10.2165/00063030-200519030-00003. [DOI] [PubMed] [Google Scholar]
- Harvey S. Growth Hormone Storage. In: Harvey S, Scanes CG, Daughaday WH, editors. Growth Hormone. CRC Press; Boca Raton: 1995. [Google Scholar]
- Harvey S. Growth Hormone Synthesis. In: Harvey S, Scanes CG, Daughaday WH, editors. Growth Hormone. CRC Press; Boca Raton: 1995. [Google Scholar]
- Heller S, Kozlovski P, Kurtzhals P. Insulin's 85th anniversary--An enduring medical miracle. Diabetes Research and Clinical Practice. 2007;78(2):149–158. doi: 10.1016/j.diabres.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Hoffman AS. Hydrogels for biomedical applications, in Bioartificial Organs III: Tissue Sourcing, Immunoisolation, and Clinical Trials. 2001:62–73. [Google Scholar]
- Hovgaard L, Jacobs H, Mazer NA, Kim SW. Stabilization of insulin by alkylmaltosides. A. Spectroscopic evaluation. International Journal of Pharmaceutics. 1996;132(1–2):107–113. [Google Scholar]
- Hurni MA, Noach ABJ, Blom-Roosemalen CM, de Boer AG, Nagelkerke JF, Breimer DD. Permeability Enhancement in Caco-2 Cell Monolayers by Sodium Salicylate and Sodium Taurodihydrofusidate: Assessment of Effect-Reversibility and Imaging of Transepithelial Transport Routes by Confocal Laser Scanning Microscopy. The Journal of Pharmacology and Experimental Therapeutics. 1993;267:942–950. [PubMed] [Google Scholar]
- Hwang S-J, Park H, Park K. Gastric Retentive Drug-Delivery Systems. Critical Reviews in Therapeutic Drug Carrier Systems. 1998;15(3):243–284. [PubMed] [Google Scholar]
- Ichikawa H, Peppas NA. Synthesis and Characterization of pH-Responsive Nanosized Hydrogels of Poly(Methacrylic Acid-g-Ethylene Glycol) for Oral Peptide Delivery. Proceedings Minutes. 2001:261–264. [Google Scholar]
- Johnson LR. Digestion and Absorption. In: Johnson LR, editor. Gastrointestinal Physiology. 6 ed. Mosby; St. Louis: 2001. [Google Scholar]
- Kararli TT. Comparison of the Gastrointestinal Anatomy, Physiology, and Biochemistry of Humans and Commonly Used Laboratory-Animals. Biopharmaceutics & Drug Disposition. 1995;16(5):351–380. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- Kashyap N, Kumar N, Kumar M. Hydrogels for pharmaceutical and biomedical applications. Critical Reviews in Therapeutic Drug Carrier Systems. 2005;22(2):107–149. doi: 10.1615/critrevtherdrugcarriersyst.v22.i2.10. [DOI] [PubMed] [Google Scholar]
- Kast CE, Bernkop-Schnurch A. Thiolated polymers - thiomers: development and in vitro evaluation of chitosan-thioglycolic acid conjugates. Biomaterials. 2001;22(17):2345–2352. doi: 10.1016/s0142-9612(00)00421-x. [DOI] [PubMed] [Google Scholar]
- Kast CE, Guggi D, Langoth N, Bernkop-Schnurch A. Development and in vivo evaluation of an oral delivery system for low molecular weight heparin based on thiolated polycarbophil. Pharmaceutical Research. 2003;20(6):931–936. doi: 10.1023/a:1023803706746. [DOI] [PubMed] [Google Scholar]
- Kavimandan NJ, Losi E, Peppas NA. Novel delivery system based on complexation hydrogels as delivery vehicles for insulin-transferrin conjugates. Biomaterials. 2006;27(20):3846–3854. doi: 10.1016/j.biomaterials.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Kim B, Peppas NA. Analysis of molecular interactions in poly(methacrylic acid-g-ethylene glycol) hydrogels. Polymer. 2003;44(13):3701–3707. [Google Scholar]
- Kissel T, Werner U. Nasal delivery of peptides: an in vitro cell culture model for the investigation of transport and metabolism in human nasal epithelium. Journal of Controlled Release. 1998;53(1–3):195–203. doi: 10.1016/s0168-3659(97)00253-8. [DOI] [PubMed] [Google Scholar]
- Klausner EA, Lavy E, Friedman M, Hoffman A. Expandable gastroretentive dosage forms. Journal of Controlled Release. 2003;90(2):143–162. doi: 10.1016/s0168-3659(03)00203-7. [DOI] [PubMed] [Google Scholar]
- Klier J, Scranton AB, Peppas NA. Self Associating Networks of Poly(methacrylic acid-g-ethylene glycol) Macromolecules. 1990;23:4944–4949. [Google Scholar]
- Knipp GT, Ho NFH, Barsuhn CL, Borchard RT. Paracellular Diffusion in Caco-2 Cell Monolayers: Effect of Perturbation on the Transport of Hydrophilic Compounds that Vary in Charge and Size. Journal of Pharmaceutical Research. 1997;86(10):1105–1110. doi: 10.1021/js9700309. [DOI] [PubMed] [Google Scholar]
- Kopeček J, Kopečková P, Brønsted H, Rahti R, Říhová B, Yeh P-Y, Ikesue K. Polymers for colon-specific drug delivery. Journal of Controlled Release. 1992;19:121–130. [Google Scholar]
- Kuzuya T, Nakagawa S, Satoh J, Kanazawa Y, Iwamoto Y, Kobayashi M, Nanjo K, Sasaki A, Seino Y, Ito C, Shima K, Nonaka K, Kadowaki T. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Research and Clinical Practice. 2002;55(1):65–85. doi: 10.1016/s0168-8227(01)00365-5. [DOI] [PubMed] [Google Scholar]
- Landin-Wilhelmsen K, Bryman I, Wilhelmsen L. Cardiac malformations and hypertension, but not metabolic risk factors, are common in Turner syndrome. Journal of Clinical Endocrinology and Metabolism. 2001;86(9):4166–4170. doi: 10.1210/jcem.86.9.7818. [DOI] [PubMed] [Google Scholar]
- Langer R, Peppas NA. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE Journal. 2003;49(12):2990–3006. [Google Scholar]
- Larhed AW, Artursson P, Gråsjö J, Björk E. Diffusion of drugs in native and purified gastrointestinal mucus. Journal of Pharmaceutical Sciences. 1997;86(6):660–665. doi: 10.1021/js960503w. [DOI] [PubMed] [Google Scholar]
- Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nature Reviews Drug Discovery. 2008;7:21–39. doi: 10.1038/nrd2399. [DOI] [PubMed] [Google Scholar]
- Lee VHL, Yamamoto A, Kompella VB. Mucosal Penetration Enhancers for Facilitation of Peptide and Protein Drug Absorption. Critical Reviews in Therapeutic Drug Carrier Systems. 1991;8:91–192. [PubMed] [Google Scholar]
- Lee YH, Sinko PJ. Oral delivery of salmon calcitonin. Advanced Drug Delivery Reviews. 2000;42(3):225–238. doi: 10.1016/s0169-409x(00)00063-6. [DOI] [PubMed] [Google Scholar]
- Leitner VM, Guggi D, Bernkop-Schnurch A. Thiomers in noninvasive polypeptide delivery: In vitro and in vivo characterization of a polycarbophilcysteine/glutathione gel formulation for human growth hormone. Journal of Pharmaceutical Sciences. 2004;93(7):1682–1691. doi: 10.1002/jps.20069. [DOI] [PubMed] [Google Scholar]
- Leitner VM, Guggi D, Krauland AH, Bernkop-Schnurch A. Nasal delivery of human growth hormone: in vitro and in vivo evaluation of a thiomer/glutathione microparticulate delivery system. Journal of Controlled Release. 2004;100(1):87–95. doi: 10.1016/j.jconrel.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Leitner VM, Walker GF, Bernkop-Schnurch A. Thiolated polymers: evidence for the formation of disulphide bonds with mucus glycoproteins. European Journal of Pharmaceutics and Biopharmaceutics. 2003;56(2):207–214. doi: 10.1016/s0939-6411(03)00061-4. [DOI] [PubMed] [Google Scholar]
- Lopez JE, Peppas NA. Effect of poly (ethylene glycol) molecular weight and microparticle size on oral insulin delivery from P(MAA-g-EG) microparticles. Drug Development and Industrial Pharmacy. 2004;30(5):497–504. doi: 10.1081/ddc-120037480. [DOI] [PubMed] [Google Scholar]
- Lowman AM, Morishita M, Kajita M, Nagai T, Peppas NA. Oral delivery of insulin using pH-responsive complexation gels. Journal of Pharmaceutical Sciences. 1999;88(9):933–937. doi: 10.1021/js980337n. [DOI] [PubMed] [Google Scholar]
- Lowman AM, Peppas NA. Analysis of the Complexation/Decomplexation Phenomena in Graft Polymer Networks. Macromolecules. 1997;30:4959–4965. [Google Scholar]
- Lowman AM, Peppas NA. Hydrogels. In: Mathiowitz E, editor. Encyclopedia of Controlled Drug Delivery. Volume 1. John Wiley & Sons; New York: 1999. [Google Scholar]
- Lowman AM, Peppas NA. Molecular analysis of interpolymer complexation in graft copolymer networks. Polymer. 2000;41(1):73–80. [Google Scholar]
- Lueβen HL, Rentel CO, Kotzé AF, Lehr CM, de Boer AG, Verhoef JC, Junginger HE. Mucoadhesive polymers and peroral peptide drug delivery. IV. Polycarbophil and chitosan are potent enhancers of peptide transport across intestinal mucosae in vitro. Journal of Controlled Release. 1997;45:15–23. [Google Scholar]
- Lyritis GP, Ioannidis GV, Karachalios T, Roidis N, Kataxaki E, Papaioannou N, Kaloudis J, Galanos A. Analgesic effect of salmon calcitonin suppositories in patients with acute pain due to recent osteoporotic vertebral crush fractures: A prospective double-blind, randomized, placebo-controlled clinical study. Clinical Journal of Pain. 1999;15(4):284–289. doi: 10.1097/00002508-199912000-00004. [DOI] [PubMed] [Google Scholar]
- Madsen F, Peppas NA. Complexation graft copolymer networks: swelling properties, calcium binding and proteolytic enzyme inhibition. Biomaterials. 1999;20(18):1701–1708. doi: 10.1016/s0142-9612(99)00071-x. [DOI] [PubMed] [Google Scholar]
- Marschutz MK, Caliceti P, Bernkop-Schnurch A. Design and in vivo evaluation of an oral delivery system for insulin. Pharmaceutical Research. 2000;17(12):1468–1474. doi: 10.1023/a:1007696723125. [DOI] [PubMed] [Google Scholar]
- Mathiowitz E, Chickering D, Jacob JS, Santos C. Bioadhesive Drug Delivery Systems. In: Mathiowitz E, editor. Encyclopedia of Controlled Drug Delivery. Volume 1. John Wiley & Sons; New York: 1999. [Google Scholar]
- Morimoto K, Katsumata H, Yabuta T, Iwanaga K, Kakemi M, Tabata Y, Ikada Y. Gelatin microspheres as a pulmonary delivery system: Evaluation of salmon calcitonin absorption. Journal of Pharmacy and Pharmacology. 2000;52(6):611–617. doi: 10.1211/0022357001774444. [DOI] [PubMed] [Google Scholar]
- Morishita M, Goto M, Takayama K, Peppas NA. Oral insulin delivery systems based on complexation polymer hydrogels. Journal of Drug Delivery Science and Technology. 2006;16(1):19–24. [Google Scholar]
- Morishita M, Goto T, Peppas NA, Joseph JI, Torjman MC, Munsick C, Nakamura K, Yamagata T, Takayama K, Lowman AM. Mucosal insulin delivery systems based on complexation polymer hydrogels: effect of particle size on insulin enteral absorption. Journal of Controlled Release. 2004;97(1):115–124. doi: 10.1016/j.jconrel.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Moriyama K, Ooya T, Yui N. Hyaluronic acid grafted with poly(ethylene glycol) as a novel peptide formulation. Journal of Controlled Release. 1999;59(1):77–86. doi: 10.1016/s0168-3659(98)00183-7. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Murray RJ, Joseph JI, Peppas NA, Morishita M, Lowman AM. Oral insulin delivery using P(MAA-g-EG) hydrogels: effects of network morphology on insulin delivery characteristics. Journal of Controlled Release. 2004;95(3):589–599. doi: 10.1016/j.jconrel.2003.12.022. [DOI] [PubMed] [Google Scholar]
- National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2005. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2005. [Google Scholar]
- Nerurkar MM, Burton PS, Borchardt RT. The Use of Surfactants to Enhance the Permeability of Peptides Through Caco-2 Cells by Inhibition of an Apically Polarized Efflux System. Pharmaceutical Research. 1996;13:528–534. doi: 10.1023/a:1016033702220. [DOI] [PubMed] [Google Scholar]
- Nicolazzo JA, Reed BL, Finnin BC. Buccal penetration enhancers--How do they really work? Journal of Controlled Release. 2005;105(1–2):1–15. doi: 10.1016/j.jconrel.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Nir Y, Paz A, Sabo E, Potasman I. Fear of Injections in Young Adults: Prevalence and Associations. Am J Trop Med Hyg. 2003;68(3):341–344. [PubMed] [Google Scholar]
- Opar A. Another blow for inhaled protein therapeutics. Nature Reviews Drug Discovery. 2008;7:189–190. [Google Scholar]
- Overgaard K, Hansen MA, Jensen SB, Christiansen C. Effect of Salcatonin Given Intranasally on Bone Mass and Fracture Rates in Established Osteoporosis - a Dose-Response Study. British Medical Journal. 1992;305(6853):556–561. doi: 10.1136/bmj.305.6853.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard K, Riis BJ, Christiansen C, Hansen MA. Effect of Salcatonin Given Intranasally on Early Postmenopausal Bone Loss. British Medical Journal. 1989;299(6697):477–479. doi: 10.1136/bmj.299.6697.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DR, Zinman B, Bolli G. Alternative routes of insulin delivery. Diabetic Medicine. 2003;20(11):886–898. doi: 10.1046/j.1464-5491.2003.01076.x. [DOI] [PubMed] [Google Scholar]
- Pade V, Stavchansky S. Link Between Drug Absorption Solubility and Permeability Measurements in Caco-2 Cells. Journal of Pharmaceutical Science. 1998;87:1604–1607. doi: 10.1021/js980111k. [DOI] [PubMed] [Google Scholar]
- Papapoulos SE. Paget's disease of bone: Clinical, pathogenetic and therapeutic aspects. Baillière's Clinical Endocrinology and Metabolism. 1997;11(1):117–143. doi: 10.1016/s0950-351x(97)80553-8. [DOI] [PubMed] [Google Scholar]
- Partanen T-M, Rissanen A. Insulin injection practices. Practical Diabetes International. 2000;17(8):252–254. [Google Scholar]
- Peppas NA. Hydrogels. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials Science. 2nd ed. Elsevier Academic Press; San Diego, CA: 2004. [Google Scholar]
- Peppas NA. Kinetics of Smart Hydrogels. In: Yui N, Mrsny R, Park K, editors. Reflexive Polymers and Hydrogels: Understanding and Designing Fast-Responsive Polymeric Systems. CRC Press; Boca Raton, FL: 2004. [Google Scholar]
- Peppas NA. Drug Delivery Using Smart Polymers: Recent Advances. In: Galaev I, Mattiasson B, editors. Smart Polymers: Applications in Biotechnology and Biomedicine. CRC Press; Boca Raton, FL: 2007. [Google Scholar]
- Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. European Journal of Pharmaceutics and Biopharmaceutics. 2000;50(1):27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- Peppas NA, Hansen PJ, Buri PA. A Theory of Molecular Diffusion in the Intestinal Mucus. International Journal of Pharmaceutics. 1984;20:107–118. [Google Scholar]
- Peppas NA, Klier J. Controlled Release by Using Poly(ethylene glycol oxide-g-methacrylic acid) hydrogels. Journal of Controlled Release. 1991;16:203–214. [Google Scholar]
- Peppas NA, Langer R. New Challenges in Biomaterials. Science. 1994;263:1715–1720. doi: 10.1126/science.8134835. [DOI] [PubMed] [Google Scholar]
- Peppas NA, Mikos AG. In: Preparation methods and structure of hydrogels. Peppas NA, editor. CRC Press; Boca Raton, FL: 1986. [Google Scholar]
- Perakslis E, Tuesca A, Lowman A. Complexation hydrogels for oral protein delivery: an in vitro assessment of the insulin transport-enhancing effects following dissolution in simulated digestive fluids. Journal of Biomaterials Science, Polymer Edition. 2007;18(12):1475–1490. [PubMed] [Google Scholar]
- Pinkerton JV, Dalkin AC. Combination therapy for treatment of osteoporosis: A review. American Journal of Obstetrics and Gynecology. 2007;197(6):559–565. doi: 10.1016/j.ajog.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Podichetty VK, Segal AM, Lieber M, Mazanec DJ. Effectiveness of salmon calcitonin nasal spray in the treatment of lumbar canal stenosis - A double-blind, randomized, placebo-controlled, parallel group trial. Spine. 2004;29(21):2343–2349. doi: 10.1097/01.brs.0000143807.78082.7f. [DOI] [PubMed] [Google Scholar]
- Ponchel G, Irache J. Specific and non-specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Advanced Drug Delivery Reviews. 1998;34:191–219. doi: 10.1016/s0169-409x(98)00040-4. [DOI] [PubMed] [Google Scholar]
- Pondel M. Calcitonin and calcitonin receptors: bone and beyond. International Journal of Experimental Pathology. 2000;81(6):405–422. doi: 10.1046/j.1365-2613.2000.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poór G, Donáth J, Fornet B, Cooper C. Epidemiology of Paget's Disease in Europe: The Prevalence Is Decreasing. Journal of Bone and Mineral Research. 2006;21(10):1545–1549. doi: 10.1359/jbmr.060704. [DOI] [PubMed] [Google Scholar]
- Portero A, Teijeiro-Osorio D, Alonso MJ, Remuñán-López C. Development of chitosan sponges for buccal administration of insulin. Carbohydrate Polymers. 2007;68(4):617–625. [Google Scholar]
- Pringels E, Callens C, Vervaet C, Dumont F, Slegers G, Foreman P, Remon JP. Influence of deposition and spray pattern of nasal powders on insulin bioavailability. International Journal of Pharmaceutics. 2006;310(1–2):1–7. doi: 10.1016/j.ijpharm.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Rabkin R, Gottheiner TI, Fang VS. Removal and Excretion of Immunoreactive Rat Growth-Hormone by the Isolated Kidney. American Journal of Physiology. 1981;240(4):F282–F287. doi: 10.1152/ajprenal.1981.240.4.F282. [DOI] [PubMed] [Google Scholar]
- Read NW, Sugden K. Gastrointestinal Dynamics and Pharmacology for the Optimum Design of Controlled-Release Oral Dosage Forms. CRC Critical Reviews in Therapeutic Drug Carrier Systems. 1988;4(3):221–263. [PubMed] [Google Scholar]
- Robinson JR. Rationale of Bioadhesion/Mucoadhesion. In: Gurny R, Junginger HE, editors. Bioadhesion - Possibilities and Future Trends. Wissenschaftliche Verlagsgesellschaft mbH; Stuttgart, Germany: 1989. [Google Scholar]
- Ross SA, Gulve EA, Wang MH. Chemistry and biochemistry of type 2 diabetes. Chemical Reviews. 2004;104(3):1255–1282. doi: 10.1021/cr0204653. [DOI] [PubMed] [Google Scholar]
- Ruppar D. Oral Delivery. Drug Delivery Technology. 2008;8(3):50–51. [Google Scholar]
- Salama NN, Eddington ND, Fasano A. Tight junction modulation and its relationship to drug delivery. Advanced Drug Delivery Reviews. 2006;58(1):15–28. doi: 10.1016/j.addr.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Salama NN, Fasano A, Thakar M, Eddington ND. The effect of Delta G on the transport and oral absorption of macromolecules. Journal of Pharmaceutical Sciences. 2004;93(5):1310–1319. doi: 10.1002/jps.20052. [DOI] [PubMed] [Google Scholar]
- Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Advanced Drug Delivery Reviews. 2005;57(11):1666–1691. doi: 10.1016/j.addr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Schipper NGM, Vårum KM, Artursson P. Chitosans as Absorption Enhancers for Poorly Absorbable Drugs. 1: Influence of Molecular Weight and Degree of Acetylation on Drug Transport Across Human Intestinal Epithelial (Caco-2) Cells. Pharmaceutical Research. 1996;13:1686–1692. doi: 10.1023/a:1016444808000. [DOI] [PubMed] [Google Scholar]
- Serra L, Domenech J, Peppas NA. Design of poly(ethylene glycol)-tethered copolymers as novel mucoadhesive drug delivery systems. European Journal of Pharmaceutics and Biopharmaceutics. 2006;63(1):11–18. doi: 10.1016/j.ejpb.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Shamlou PA, Breen LH, Bell WV, Pollo M, Thomas BA. A new scaleable freeze-thaw technology for bulk protein solutions. Biotechnology and Applied Biochemistry. 2007;46:13–26. doi: 10.1042/BA20060075. [DOI] [PubMed] [Google Scholar]
- Sinha VR, Trehan A. Biodegradable microspheres for protein delivery. Journal of Controlled Release. 2003;90(3):261–280. doi: 10.1016/s0168-3659(03)00194-9. [DOI] [PubMed] [Google Scholar]
- Sipahigil O, Gursoy A, Cakalagaoglu F, Okar IT. Release behaviour and biocompatibility of drug-loaded pH sensitive particles. International Journal of Pharmaceutics. 2006;311(1–2):130–138. doi: 10.1016/j.ijpharm.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Song Y, Wang Y, Thakur R, Meidan VM, Michniak B. Mucosal Drug Delivery: Membranes, Methodologies, and Applications. Critical Reviews in Therapeutic Drug Carrier Systems. 2004;21(3):195–256. doi: 10.1615/critrevtherdrugcarriersyst.v21.i3.20. [DOI] [PubMed] [Google Scholar]
- Swenson EC, Curatolo WJ. Intestinal Permeability Enhancement for Proteins, Peptides and Other Polar Drugs: Mechanisms and Potential Toxicity. Advanced Drug Delivery Reviews. 1992;8:39–92. [Google Scholar]
- Tanaka Y, Taki Y, Sakane T, Nadai T, Sezaki H, Yamashita S. Characterization of Drug Transport Through Tight-Junctional Pathway in Caco-2 Monolayer: Comparison with Isolated Rat Jejunum and Colon. Pharmaceutical Research. 1995;12:523–528. doi: 10.1023/a:1016245711557. [DOI] [PubMed] [Google Scholar]
- Thomas JB, Tingsanchali JH, Rosales AM, Creecy CM, McGinity JW, Peppas NA. Dynamics of poly(ethylene glycol)-tethered, pH responsive networks. Polymer. 2007;48(17):5042–5048. doi: 10.1016/j.polymer.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Lugo M, García M, Record R, Peppas NA. Physicochemical Behavior and Cytotoxic Effects of P(MAA-g-EG) Nanospheres for Oral Delivery of Proteins. Journal of Controlled Release. 2002;80:197–205. doi: 10.1016/s0168-3659(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Torres-Lugo M, Garcia M, Record R, Peppas NA. pH-sensitive hydrogels as gastrointestinal tract absorption enhancers: Transport mechanisms of salmon calcitonin and other model molecules using the Caco-2 cell model. Biotechnology Progress. 2002;18(3):612–616. doi: 10.1021/bp0101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Lugo M, Peppas NA. Molecular design and in vitro studies of novel pH-sensitive hydrogels for the oral delivery of calcitonin. Macromolecules. 1999;32(20):6646–6651. [Google Scholar]
- Total Prevalence of Diabetes & Pre-diabetes. American Diabetes Association. [cited 24 April 2008]; Available from: http://www.diabetes.org/diabetes-statistics/prevalence.jsp.
- Türker S, Onur E, Ózer Y. Nasal route and drug delivery systems. Pharmacy World and Science. 2004;26(3):137–142. doi: 10.1023/b:phar.0000026823.82950.ff. [DOI] [PubMed] [Google Scholar]
- Uchida T, Sakakibara S, Toida Y, Nagareya N, Yasuda N, Matsuyama K. Rectal Delivery of Insulin Using a Bioadhesive Hydrogel Preparation Containing Lauric Acid as an Enhancer in Rats. Pharmacy and Pharmacology Communications. 1999;5:523–527. [Google Scholar]
- Ugwoke MI, Verbeke N, Kinget R. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. Journal of Pharmacy and Pharmacology. 2001;53(1):3–21. doi: 10.1211/0022357011775145. [DOI] [PubMed] [Google Scholar]
- Wehren LE. The role of the primary care physician in diagnosis and management of osteoporosis. International Journal of Fertility and Womens Medicine. 2002;47(3):116–122. [PubMed] [Google Scholar]
- Werle M. Natural and synthetic polymers as inhibitors of drug efflux pumps. Pharmaceutical Research. 2008;25(3):500–511. doi: 10.1007/s11095-007-9347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werle M, Bernkop-Schnurch A. Inhibition of Enzymes and Secretory Transport. In: Touitou E, Barry BW, editors. Enhancement in Drug Delivery. Taylor & Francis; Boca Raton: 2006. [Google Scholar]
- Wood KM, Stone GM, Peppas NA. Wheat germ agglutinin functionalized complexation hydrogels for oral insulin delivery. Biomacromolecules. 2008;9(4):1293–1298. doi: 10.1021/bm701274p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H-B, Huang K-X, Zhu Y-S, Gao Q-H, Wu Q-Z, Tian W-Q, Sheng X-Q, Chen Z-X, Gao Z-H. Hypoglycaemic Effect of a Novel Insulin Buccal Formulation on Rabbits. Pharmacological Research. 2002;46(5):459–467. doi: 10.1016/s1043661802002049. [DOI] [PubMed] [Google Scholar]