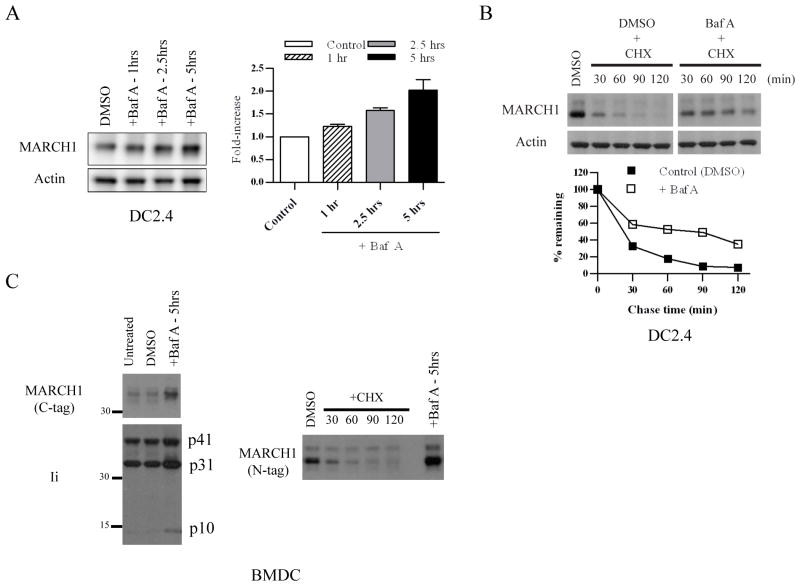
Figure 2. Lysosome-dependent turnover of MARCH1.
A. DC2.4+HA-MARCH1 cells were incubated with Bafilomycin A (Baf A) or diluent (DMSO) for 1, 2.5, or 5 hours, and then cell lysates were blotted for MARCH1 and actin. The band intensities were quantified from four independent experiments, normalized to actin, and graphed as the fold-increase over control (DMSO) +/− SEM. B. DC2.4+MARCH1 cells were pre-treated for 0.5 hours with Baf A (or DMSO). Then, cycloheximide-chase was performed as described in the Figure 1 legend. In this case, Baf A and cycloheximide were present during the chase period. Note: MARCH1 blot samples were run on the same gel, and the gel was split to remove irrelevant lanes. The band intensities were quantified, normalized to actin, and graphed as the percent-remaining as compared to time zero (DMSO-treated samples) +/− SEM. Results are representative of four experiments. C. BMDC were infected with lentiviral vectors encoding HA.MARCH1 or MARCH1.myc (C-terminal tag). Experiments were performed three days post-infection. Left panels = infected cells were treated with Baf A (or DMSO control) and blotted for the myc-tagged MARCH1 and for invariant chain (Ii). Right panels = BMDC were transduced with HA-MARCH1, and cycloheximide-chase and immunoblot of MARCH1 were performed as described in the legend of Figure 1. Right lane represents the same cells treated for 5 hours with Baf A.