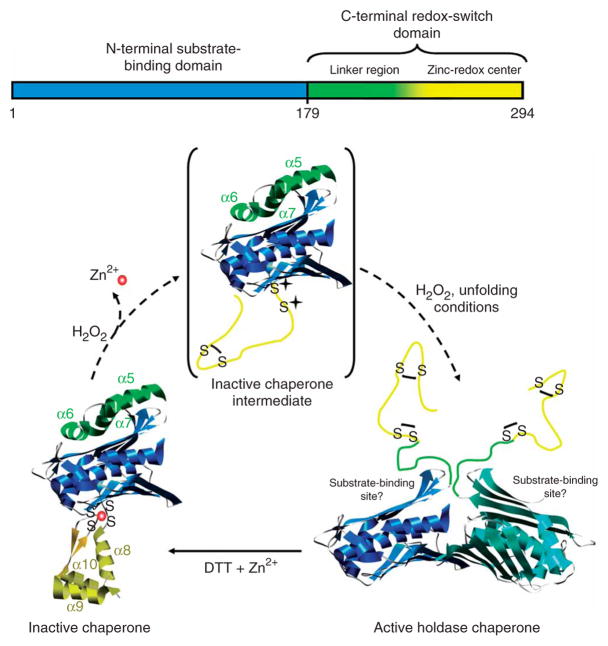
Figure 7.
Schematic model of Hsp33’s activation process. Upon oxidation of Hsp33 at 30 °C, zinc is released and the zinc center (α-helices 8–10) unfolds. This unfolding is apparently not sufficient to activate the chaperone function, but generates an oxidation intermediate of Hsp33 (shown in brackets), which is presumably only very transiently populated during oxidation at 43 °C. Upon exposure to oxidizing and unfolding conditions—for example, H2O2 at elevated temperature—the complete C terminus (α-helices 5–10) converts to a natively unfolded protein. These extensive conformational rearrangements lead to the exposure of hydrophobic surfaces, presumably on the N-terminal substrate-binding domain of Hsp33, and allow Hsp33 to dimerize.