Table 1.
Oxidative cleavage of aliphatic epoxides
 | |||||
|---|---|---|---|---|---|
| Entry yieldb | Substrate | Product | Solvent | NaIO4a (equiv) | |
| 1 |
|
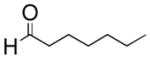
|
CH3CN/H2O | 5 | 73% |
| 2 |

|

|
CH3CN/H2O | 5 | 58%c |
| 3 |

|

|
CH3CN/H2O | 4 | 78% |
| 4 |

|

|
THF/H2O | 2 | 67% |
| 5 |
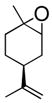
|
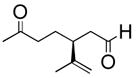
|
THF/H2O | 2 | 91% |
| 6 |

|

|
THF/H2O | 2 | 88% |

|

|
||||
| 7 | X = H | CH3CN/H2O | 2 | 84% | |
| 8 | X = p-Cl | CH3CN/H2O | 2 | 34% | |
| 9 | X = p-F | THF/H2O | 2 | 62% | |
| 10 | X = o-Br | CH3CN/H2O | 2 | 71%d | |
| 11 |

|

|
CH3CN/H2O | 2 | 41%e |
| 12 |

|

|
CH3CN/H2O | 2 | 64% |
A significant drop in pH occurred with the addition of NaIO4 to the THF/H2O mixture (pH 6 to pH 4).
Isolated yield.
Starting material was recovered in 39% yield.
Based on recovered starting material.
Only PhCHO was observed.
