Figure 7.
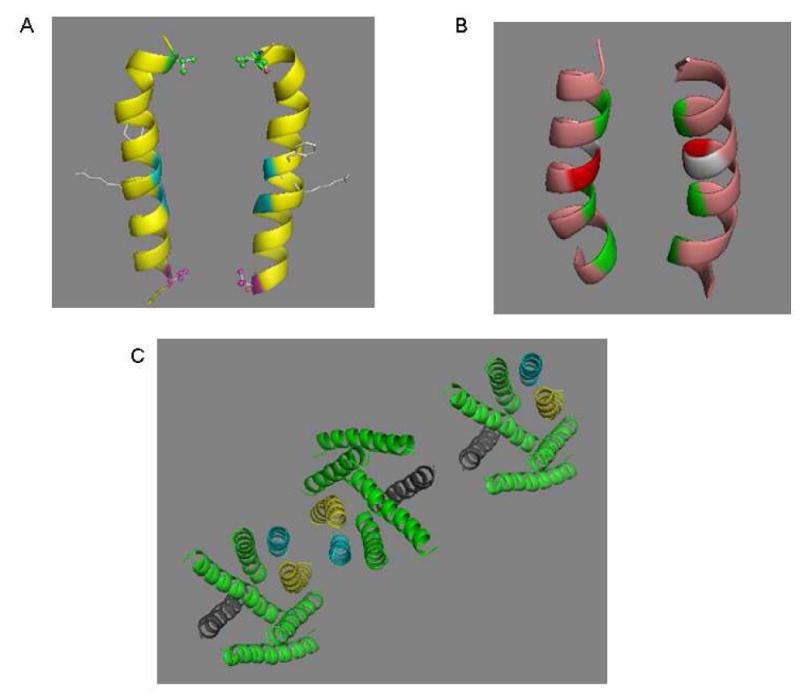
Dimerization interfaces of Ste2p. A. V68 (red), a dimer contact found in the current study, is located on the same plane with V45 (green) a dimer contact at the extracellar end of TM1 (21). The backbones of two glycine residues (blue) known to play a role in Ste2p dimerization are shown. Two resdues (F55, R58) involved in ligand binding are presented as a stick model located on the opposite side of the dimer interface. B. TM7/TM7 dimer interface. The backbone of three dimer contacts identified from the current study (T278, A285 and L289, from top to bottom) are colored green. A281 (light gray) and T282 (red) would appear to be candidates for dimer contact based on a sideview of TM7 (Fig. 8A), but the model here shows that alignments of these two residues are not favorable for disulfide bond formation. C. Proposed oligomerization interfaces of Ste2p. Dimerization of Ste2p involving TM1/TM1 (magenta) and/or TM7/TM7 (yellow) interface allows TM4/TM4 interface (dark grey) to mediate a new dimerization contact enabling oligomerization. An extracellular view of a representative trimer is shown.
