SUMMARY
Phosphomannomutaste (PMM2, Mannose-6-P→ Mannose-1-P) deficiency is the most frequent glycosylation disorder affecting the N-glycosylation pathway. There is no therapy for the hundreds of patients who suffer from this disorder. This review describes previous attempts at therapeutic interventions and introduces perspectives emerging from the drawing boards. Two approaches aim to increase Mannose-1-P: small membrane permeable molecules that increase the availability or/and metabolic flux of precursors into the impaired glycosylation pathway; and, phosphomannomutase enhancement and/or replacement therapy. Glycosylation-deficient cell and animal models are needed to determine which individual or combined approaches improve glycosylation and may be suitable for preclinical evaluation.
Keywords: Glycosylation disorder, CDG, phosphomannomutase2, phosphomannose isomerase, therapy, high throughput screening
INTRODUCTION
Inherited deficiency of phosophomannomutase (PMM2) causes a human glycosylation disorder previously known as Congenital Disorder of Glycosylation Ia (CDG-Ia) [1]. Pediatric patients have a broad and variable clinical picture that affects nearly all systems leading to failure to thrive, hypotonia, variable developmental delay, ataxia, dysmorphia, skeletal abormalities, coagulopathy with up to 20% mortality in the first 5 years due to organ failure and/or severe infections [2–5]. Later on they show mental retardation, stroke like episodes, and retinitis pigmentosa. Adults are usually wheelchair bound with peripheral neuropathy and stable mental retardation. The spectrum is broad and recently a considerable number of very mild patients have been reported [6–10]. There is no therapy for >600 identified patients; many more patients probably remain undetected. Scores of mutations have been identified in PMM2-deficient patients. Some of these patients have up to 25% normal enzymatic activity, while heterozygous parents with 50% activity are asymptomatic [9]. This surprisingly high threshold raises the question: how much enzymatic activity is required to prevent pathology? Since no precise genotype-phenotype correlation is seen [11], an ensemble of other unidentified genes must play important roles. However, patients with high residual activity tend to have milder phenotypes [11, 12]. While developmental defects that occur in utero cannot be reversed, even a small improvement in the effective enzymatic activity might reduce the daily medical burden on patients and their families. This hope makes the pursuit of a therapy worthwhile.
The purpose of this article is to review previous attempts at therapy for PMM2- deficient patients and to present the rationale for the development of new ones.
METABOLIC PATHWAYS
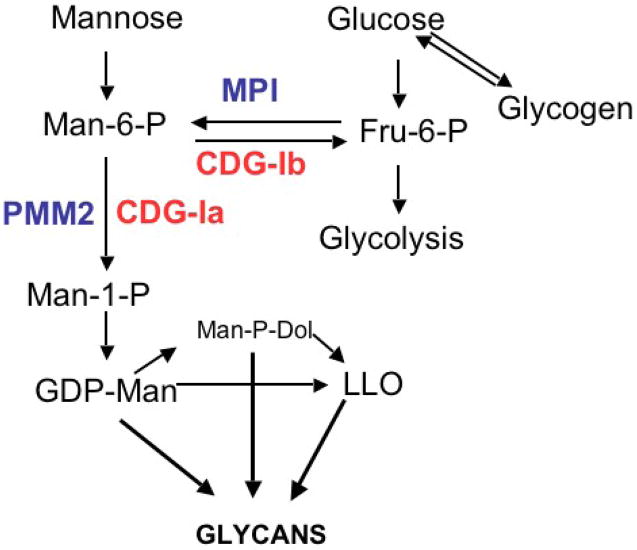
PMM2 is an essential gene and its complete loss is lethal in yeast, mice and presumably humans [13, 14]. PMM1 is a homolog of PMM2 [15], and catalyzes the same reaction, but it does not appear to substitute for loss of PMM2 in mice [16]. PMM1 ablation in mice produces no obvious pathology or phenotype [16]. Recent studies show that this enzyme, which is highly expressed in brain, is an IMP-stimulated glucose1,6-P phosphatase [17] PMM2 catalyzes the conversion of Mannose-6-P (Man-6-P) into Mannose-1-P (Man-1-P) and it is the first committed step in synthesis of the activated mannose donors GDP-mannose and dolichol-P-Mannose, as shown in Figure 1. Synthesis of N-glycans, O-mannose linked glycans, glycophosphotidylinositol anchors, and C-mannosylated proteins all require these donors. Cells transport exogenous mannose and convert it to Man-6-P using hexokinase. Alternatively, Man-6-P is formed from Fructose-6-P using phosphomannose isomerase (PMI or MPI). Both exogenous glucose and glycogen-derived glucose can contribute to this pathway [18, 19]. The majority of mannose in N-glycans is derived from glucose in most cells (Freeze and Sharma, unpublished observations). Deficiencies in PMM2 and MPI reduce the amount of Man-1-P and Man-6-P [20, 21], respectively, that are available for glycosylation, resulting in unoccupied N-linked glycosylation sites on a number of proteins. The absence of glycan chains is thought to cause the pathology seen in these patients. Excess Man-6-P derived from mannose is catabolized beginning with PMI. All of the biosynthetic reactions are thought to occur in the cytoplasm or on the cytoplasmic face of the endoplasmic reticulum.
Figure 1. Mannose-related Metabolic Pathways in Glycan Synthesis.
PMM2 deficiency, the cause of CDG-Ia, affects the first committed step required for the synthesis of mannosylated glycans. Reduced production of Man-1-P reduces its flux through the pathway and leads to reduced N-glycosylation. Whether other pathways are affected is not known. The goal of therapy is to increase the amount of Man-1-P available for glycosylation. This can be done by providing Man-1-P directly or by increasing the flux of Man-6-P to Man-1-P. Providing normal PMM2, stabilizing or activating the endogenous enzyme or driving more Man-6-P into the pathway may increase the amount of Man-1-P and improve impaired glycosylation. See text for a detailed discussion.
SETTING THE STAGE FOR THERAPY: WHAT IS POSSIBLE AND PRACTICAL?
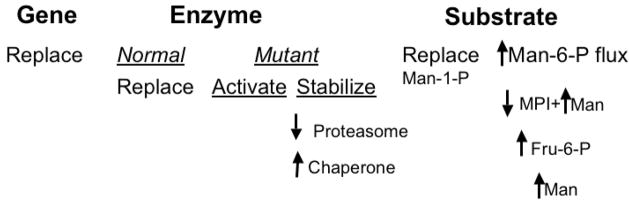
Cellular, genetic, and metabolic options should be considered to treat PMM2-deficient patients. Some are more practical than others and/or may be achievable within a reasonable timeframe; but all have shortcomings. See Table 1 and Figure 2 for an overview and summary. Applications of stem cell therapy are in the far future [22]. While they may hold promise for treating inherited liver disorders [23, 24] or those disorders amenable to hematopoietic stem cell replacement [25, 26] treating systemic glycosylation disorders would be considerably more difficult because of the ubiquitous and cell-autonomous nature of protein glycosylation. This approach will not be considered here in favor of near term options and better targets. The goal of therapy for PMM2-deficient patients is to increase the flux of metabolic precursors into the impoverished glycosylation pathways. Options include: increase the activity of PMM2, provide Man-1-P directly, increase the Man-6-P pool and/or redirect this substrate from glycolysis to glycosylation. Enzyme activity could be increased by gene therapy that included stem cells, but it is also unlikely to be useful in the near future, given the inherent risks and very small patient population [22]. Enzyme replacement therapy has not been tried and insulin is the only reported activator of PMM2 [27]. Man-1-P is not membrane permeable, but hydrophobic derivatives can enter cells and correct impaired glycosylation. However, these compounds tend to be unstable and toxic. Increasing exogenous mannose can improve [3H] mannose incorporation in PMM2-deficient cells, but mannose therapy was not effective in patients.
Figure 2. Summary of Potential Approaches for Treating PMM2-Deficient Patients.
Therapuetic options include replacement of the gene, PMM2, supplementation with normal enzyme PMM2, or altering the stability or activity of the mutant enzyme. In addition, the substrate (Man-1-P) can be enhanced by direct replacement or alterations of the metabolic flux into the glycosylation pathways. These options are discussed in the text.
MANNOSE SUPPLEMENTS: SIMPLE BUT INEFFECTIVE
MPI-deficient patients (CDG-Ib) benefit from alimentary addition of 300–750 mg/kg/day Man [12, 28] because it bypasses the defective step by allowing formation of Man-6-P via hexokinase (Figure 1). These children fail to thrive, coagulopathies, protein-losing enteropathy and liver fibrosis, and they show remarkable improvement when the therapy is initiated [12, 29]. Fortunately, there are no neuronal pathologies in this disorder. Possibly mannose in mother’s plasma provides an “endogenous therapy” during fetal life.
In contrast, mannose treatment in PMM2-deficient CDG-Ia patients was not successful. There was no measurable improvement in any clinical parameters in children during treatment [30, 31] nor was serum hypoglycosylation decreased. The original basis for suggesting the treatment was that PMM2-deficient fibroblasts synthesize a truncated LLO glycan, which becomes normal when cells are incubated with high concentrations of mannose [32]. The most likely explanation for the failure of mannose therapy in the patients was that Man-6-P resulting from increased mannose was not available because it was simply catabolized via MPI, since its activity remains normal in PMM2-deficient patients.
Another explanation for the failure of mannose therapy in these patients was suggested based on observations using streptolysin O-permeabilized cells [33, 34]. These studies showed that addition of Man-6-P to cells preferentially reduced the amount of the major LLO species, Glc3Man9GlcNAc2-PP-Dol resulting in the liberation of the free glycan. Thus, increasing Man-6-P in either PMM or MPI deficient cells could potentially decrease the amount of available LLO and actually exacerbate deficient glycosylation. However, providing mannose to either PMM2- or MPI-deficient cells increases the deficient GDP-Mannose pools [35], showing that the metabolic flux increased, but it did not increase either the Mannose-6-P pool or decrease cellular glycosylation [36]. In contrast, providing mannose to intact Mpi-null mouse fibroblasts greatly increases Man-6-P pools and eventually depletes ATP stores, since there is no metabolic exit for Man-6-P after saturating the glycosylation pathway [37]. Increased Man-6-P in Mpi-null cells decreased the amount Glc3Man9GlcNAc2-PP-Dol LLO, but it did not appear to affect the amount of protein glycosylation [36]. This suggests that the amount of LLO is in excess. Changes in steady state level do not necessarily reflect the amount of flux through the pathway. It is important to distinguish between these.
BYPASS THE DEFECT: MEMBRANE PERMEABLE MANNOSE-1-P
Another potential approach is to provide Man-1-P directly to cells. However, Man-1-P does not diffuse, and is not transported across the cell membrane. To overcome this issue, several groups attempted to chemically synthesize cell permeable Man-1-P compounds that would be converted to free Man-1-P after entering the cell, i.e., prodrugs [38, 39]. A series of compounds have been reported [38–40]. One set showed some biological activity [38]. Man-1-P can be made membrane permeable by covering phosphates with acetoxymethyl groups and protecting the OH-groups with ethylcarbonate or acetyl esters [38]. In cell culture, these compounds correct the LLO phenotype in several glycosylation-deficient fibroblasts at a lower concentration than Man, and also compete with radiolabeled Man in a glycosylation assay in cells [38]. The study also showed that the level of GDP-Man increased in response to the compounds. This validates the theoretical concept. However, all compounds synthesized to date are far too unstable to be clinically useful. The best compound has a half-life in serum of about 2.5 minutes. This is further confounded by the relatively high concentrations needed to get sufficient uptake through the cell membrane. The best compounds must be added to the medium at a high concentration of around 100 μM to be effective. A third issue is the toxicity of these compounds. The most effective compound synthesized so far shows negative effects on protein synthesis and cell viability at concentrations above 100 μM, i.e. in the same range as its therapeutic dosage. In a more recent study [40], Man-1-P pro-drugs containing an additional phosphodiester-linked mannose residue along with a benzyl or phenyl group on the phosphate were reported to generate intracellular Man-1-P based on their ability to compete with 3H-mannose for glycosylation. The compounds were also reported to have minimal toxicity after 16 hours based on release of lactate dehydrogenase (cell lysis). Additional studies are needed to confirm and extend these observations. Using less toxic blocking groups [41], or lipid carriers for delivery of free Man-1-P is another. Apart from the labor-intensive synthesis of making a stable, non-toxic and well-transported compound, no animal study system is currently available. All PMM2-deficient patients have some residual enzymatic activity, but complete elimination of Pmm2 in mice is lethal in early embryogenesis [14]. This is also true for the Mpi-null mouse [37]. Model systems with quantifiable phenotypes will be needed to assess any therapy.
ENZYME REPLACEMENT THERAPY
Enzyme replacement therapy might be possible for PMM2-deficient patients. This approach has worked well for patients with lysosomal storage diseases. Gaucher’s patients who are deficient in glucocerebrosidase are treated with Cerezyme a form of the expressed enzyme carrying Mannose-terminated N-glycan chains that targets the enzyme to macrophage in the liver spleen and skeleton the undegraded glucosecerebroside (Glcβ-Cer) accumulates [42–44]. The enzyme gradually reduces the accumulation of the substrate since it is trafficked to its normal site of action in the lysosome [42–44]. Enzyme replacement therapy has also been approved for other lysosomal storage disorders including Pompe Disease (α-glucosidase deficiency) and MPS1 (α-iduronidase deficiency). Many of these patients have very low residual enzymatic activity (a few percent) and even slight increases of activity within the lysosome may be sufficient to improve patients’ symptoms gradually as the accumulated substrate decreases. This may not true for PMM2-deficient patients where slight increases in activity might still be within the classical pathological range. Moreover, targeting PMM2 to the lysosome is not desirable, since it would likely be degraded; it needs to remain in the cytoplasm.
Many studies show that coupling arginine-rich peptides to high molecular mass cargos delivers them into cells, through an uptake system that probably involves multiple receptors, [45] and this has proven to work in rodents in vivo [46]. Whether sufficient PMM2 could enter cells and reside in the cytoplasm with an attached cationic sequence is not known. A slightly modified approach may offer another opportunity by binding cationic-derivitized molecules to heparan sulfate on the cell surface [47]. By converting all ammonium groups of the antibiotic neomycin to guanidinium groups, molecules >300 kDa could cross cell membranes at nanomolar transporter concentrations with delivery being entirely dependent on cell surface heparan sulfate proteoglycans. By conjugating the guanidinoneomycin to the ribosome-inactivating toxin, saporin, the study showed proteoglycan-dependent delivery of cargo into the cytoplasm. Coupling of typical arginine-rich peptides showed both heparan sulfate-dependent and -independent cellular uptake [47]. Since cell surface heparan sulfate is nearly ubiquitous, this presents an opportunity to deliver PMM2 to these cells.
The disadvantage of enzyme replacement therapy is the limited amount of enzyme that can be delivered to a cell/tissue. Another is that the enzymes are unlikely to cross the blood brain barrier providing limited benefit for brain and central nervous system pathologies.
ACTIVATE OR STABILIZE PMM2
The only reported activator of PMM2 is an insulin-dependent 2-fold increase in PMM2 activity seen in Cos7 cells over expressing the enzyme. Transcription does not increase. Rather, it seems to involve a serum and glucocorticoid-regulated kinase (Sgk1), which regulates channels and transporters including the renal epithelial Na(+) channel (ENaC) [48]. In vitro phosphorylation of PMM2 by this kinase completely eliminates activity both in the absence and in the presence of insulin. The interaction with PMM2 was observed in a yeast two-hybrid screen using Sgk1 as bait, and was confirmed by co-immunoprecipitation and Sgk1-dependent in vitro phosphorylation of PMM2. These data suggest that Sgk1 may affect glycosylation, but the physiological relationship is not clear.
There are no small molecule activators of PMM2, but the availability of large chemical libraries and robotic high throughput screening facilities offer a robust opportunity to explore this avenue. The simple coupled enzymatic assay using MPI, phosphoglucose isomerase and glucose-6-P dehydrogenase can be easily adapted for compound screening. Initial leads and structural optimization of selected chemical scaffolds can be combined with docking studies to further refine and optimize the structure activity relationships activators. The structure of PMM2 has not been solved, but the structure of its homolog, PMM1, was determined by X-ray crystallography [49]. The structure suggested a likely, but complex, reaction mechanism involving cap and core domains, which open to bind substrate and then close to provide a solvent-exclusive environment for catalysis. The substrate first binds to the cap and then is swept into the active site when the cap closes. Repulsion of positive charges at the interface of the cap and core domains stabilizes alpha-PMM1 the open conformation, but negatively charged substrate binds to the cap, thereby facilitating its closure over the core domain. A potential allosteric or conformational-dependent activator could be tested initially using normal PMM2, but its activity must also be confirmed on PMM2 carrying several common mutations to select the compounds with broadest application. A small molecule could potentially pass the blood brain barrier. A disadvantage of the approach is the possibility that effective inhibitors are more effective on some mutant forms than on others, which may limit their general application.
Enzyme enhancement therapy has shown some success in treating lysosomal storage disorders [42, 43]. This approach exploits the well-known fact that substrates or their analogs stabilize enzymes. Competitive inhibitors bind at or near the active site of the mutated enzymes at neutral pH and are then released at low pH when the enzyme arrives in the lysosome. These molecules may only marginally increase the residual activity up to a few percent of normal, but this is sufficient to reduce the ongoing accumulation of non-degraded substrates. A competitive inhibitor is probably not useful for PMM2 because its synthesis and continued residence in the cytoplasm precludes a pH-dependant inhibitor dissociation to allow substrate binding. Identification of an allosteric activator, as described above, has a greater chance of success. Recently, a combination of protein specific pharmacologic chaperones and induction of the protein folding machinery synergized to allow mutated proteins to survive within the secretory pathway [50]. This approach may hold promise for ER or Golgi associated proteins that are mutated in other types of glycosylation disorders, but similar approach for PMM2 would require its stabilization and dampening of cytoplasmic protein degradation.
INCREASE MANNOSE-6-P FLUX INTO GLYCOSYLATION PATHWAYS
It should be possible to encourage the flux of Man-6-P toward the glycosylation pathways in PMM2-deficient cells. Metabolic labeling with [3H] Mannose showed these cells synthesize truncated LLO species and transfer them to proteins [32]. Addition of 250–500uM mannose to the culture medium, normalizes both the LLO pattern and the size of protein-bound glycans. Hyperphysiological (severe diabetic) concentrations of glucose (>10mM) also corrected the size, but clearly mannose was >20 fold more effective [32]. Since mannose is well tolerated at 4–5x the normal plasma concentration of 50uM, it would be reasonable to increase plasma mannose concentration. As mentioned above, mannose is not effective in PMM2-deficient patients, most likely because MPI is fully active and consumes the Man-6-P. The unfavorable ratio of PMM:MPI activity does not favor glycosylation. Increasing mannose delivery to the cells either by increasing extracellular mannose concentration or by improving its delivery into the cells might improve this situation. The antidiabetic drug, metformin, which stimulates the AMP-activated protein kinase, was reported to provide a preferential 2-fold stimulus of mannose uptake into fibroblasts [51]. However, this stimulation is not seen at normal plasma glucose concentration (5mM), so it is unlikely to increase mannose delivery in normal conditions. Another way to increase Man-6-P is by increasing the flux of glucose into fructose-6-P via glycolysis. One study showed that 5-aminoimidazole-4-carboxamide riboside (AICAR), which activates glycogen phosphorylase and suppresses glucose-6-phosphatase transcription [52] can provide mannose for glycan synthesis [34, 53]. Other studies also show that glycogen is a source of mannose in the plasma [19] AICAR was found to prevent fat accumulation in sedentary mice and was suggested as a way to increase endurance without exercise [54]. PMM2-deficient patients have normal plasma glucose and apparently normal glycogen stores. For unexplained reasons, they usually show a severe failure to thrive as infants, and as children, they struggle to gain weight, and are sometimes fed through gastrostomy tubes. Use of AICAR as a way to increase intracellular Man-6-P in PMM2-deficient patients would require caution.
An approach, which we have pursued, hinges on the observation that the amount of exogenous mannose directed toward glycan synthesis (PMM2-dependent) vs. catabolism (MPI-dependent) is determined by the ratio of PMM:MPI. Higher PMM2 activity, and lower MPI activity, drives more Man-6-P into the glycosylation pathway. Combining MPI inhibitors with hyper-physiological concentrations of mannose may increase Man-6-P flux in favor of glycosylation.
MPI is a zinc-dependent enzyme and so typical chelators such as 1,10-phenathroline, EDTA and 2,2′-dipyridyl are effective inhibitors [55, 56]. Competitive inhibitors include phosphorylated analogs such as arabinose-5-phosphate, erythrose-4-P, and ribose-5-P, but none of these are specific or membrane-permeable. Fructose-1-P was reported to inhibit MPI and this was offered as an explanation for why patients with uncontrolled hereditary fructose intolerance make hypoglycosylated proteins [57]. Previous screening of a combinatorial chemical library identified a membrane permeable compound, but its biological effects were uncharacterized [58]. The crystal structure of Candida albicans MPI is known [59], and software programs can model docking of substrate and/or potential competitors.
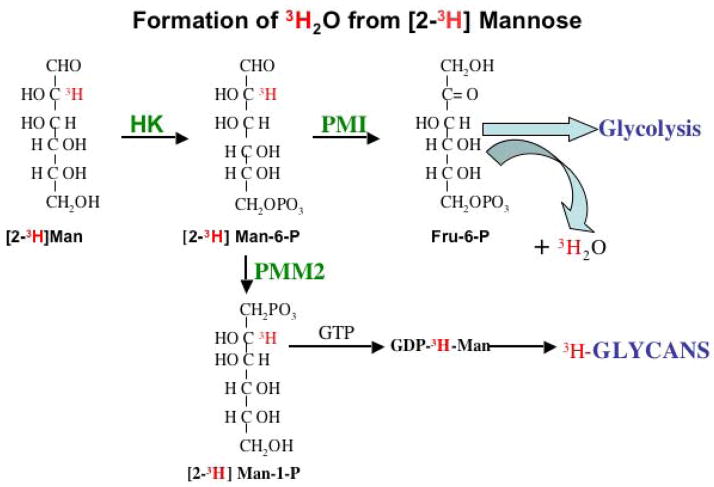
We used high throughput screening of chemical libraries to identify a first generation of MPI inhibitors [60]. The traditional MPI-coupled enzyme assay leading to NADPH+ formation was used, along with counter screens to eliminate effects on the coupling enzymes (phosphoglucose isomerase and G6PD) are required for production of NADPH+ [55]. After identifying possible inhibitors, dose-response, and IC50 values for either competitive or (preferably) non-competitive inhibitors were done using the coupled assays. Direct in vitro confirmation is easy using only MPI, based on formation of 3HOH from [2-3H]Man-6-P (Figure 3) and expressed enzyme [28, 37, 61]. This simple assay can be directly applied to cells to assess the biological effectiveness of compounds by comparing the ratio of [2-3H]mannose incorporation into glycans (TCA precipitate) vs. 3HOH production. The inhibitor should increase the incorporation of label into glycans and decrease the amount of 3HOH produced. This method can be used to assess the effects of inhibitors in more complex models such as mice or zebrafish. Previous studies using mice and most cell lines show that the great majority of [2-3H] mannose is catabolized via MPI, with production of 3HOH ([61, 62]; and unpublished observations). Increased organ specific incorporation of label into glycans would indicate that the MPI–specific inhibitors have been effective at that location. An inhibitor should decrease the amount or rate of 3HOH appearance in the plasma, but the organ/tissue that produced it could not be determined.
Figure 3. Metabolism of [2-3H] Mannose: the metabolic fate of exogenous mannose.
This versatile radiolabel is the best indicator of mannose flux into these pathways.
We screened ~200,000 compounds and identified a series of compounds with IC50’s in the range of 1–5μM. Tests on a multiple cell lines showed that, only a few of these increased [2-3H] mannose incorporation into proteins and lowered formation of 3HOH. The increased incorporation is consistent with about a 70% reduction MPI activity. Commercially available and custom-synthesized analogs are being interrogated in order to increase the efficiency of inhibition.
Such inhibitors have limitations. Besides the obvious caveats of off-target effects, toxicity, and short half life, inhibitors may have limited access to some tissues or only be effective in selected tissues where the normal ratio of PMM:MPI is favorable. For instance in the mouse, the MPI specific activity in liver was measured at about 15 nmole/min/mg protein, but it is nearly 60 nmole/min/mg in heart and ~120 nmole/min/mg in testis. It may be more challenging to reduce MPI activity sufficiently to drive the elevated plasma mannose into the glycosylation pathway in these tissues with high MPI activity.
FUTURE PERSEPCTIVES AND CHALLENGES
Individual or combinations of PMM2 activators, MPI inhibitors+plasma mannose, and PMM2 replacement may offer hope to improve glycosylation in some PMM-deficient patients. Small molecules may traverse the blood brain barrier, but PMM2 will not. On the other hand, the half-life of PMM2 may be significantly longer than that of either inhibitors or activators. Differential accessibility of small molecules and of PMM2 are significant hurdles to overcome. Since PMM2- deficiency affects so many organ systems in acute, chronic, and progressive ways, it is worthwhile to consider simultaneous exploration of all these approaches. It is difficult to predict which aspects of PMM2-deficient patients’ phenotype would respond to therapy.
The major limitation on development of these therapies is the lack of appropriate model systems, since the knockout mouse is lethal [14]. It is unlikely that organ- or temporal-specific knockout expression, e.g., floxed alleles, could be generally useful, but this would depend on the organ-to-organ variability in excision efficiency. Moreover, each cell is responsible for generation of its own Man-1-P and elimination of all PMM2 activity would preclude analysis of Pmm2 activators or Mpi inhibitors. Knock-in of human disease-causing PMM2 mutations may offer hope, although animals with observable phenotypes must be generated while avoiding embryonic or early post-natal lethality. A construct that allowed temporal or organ-specific elimination of the normal allele and simultaneous expression of the selected mutation might provide a useful model. Another approach might be to identify PMM2 shRNAi molecules that give 80–90% reduced enzymatic activity and stably integrate them into 2–4 cell embyros via lentiviral constructs [63]. This procedure could yield individuals for phenotypic analysis, but these viral constructs frequently integrate into multiple sites on different chromosomes [63], which would complicate the creation of a stable knockdown line. Nevertheless, such experiments may provide information on a range of phenotypic abnormalities in a series of individual mice. Zebrafish present another opportunity to test the therapeutic agents since the morpholino mutants produce hypomorphic alleles, similar to patients [64, 65]. This system offers great promise. A non-genetic approach to generate a PMM2-deficient model system would be to identify a specific inhibitor of PMM2. This could easily be done in the same high throughput screen searching for a therapeutic PMM2 activator. A rather different approach would be to reduce the impact of hypoglycosylation of key proteins as suggested by Shang et al [66]. They propose that tailoring the rate of protein synthesis to impaired flux through the glycosylation pathway might be useful
CONCLUSIONS
In the last decade we have made great progress in identifying a few dozen human glycosylation disorders [2, 4]. We have made almost no progress in providing the patients and families with potential therapies. The advent of high throughput compound screening offers a new avenue for development of therapies targeting the N-glycosylation pathway. The technology provides tools and reasonable approaches to assess the efficacy of potential lead compounds. Robust PMM2 deficiency models are needed to test these concepts and molecules before they will lead to useful products. International cooperation and collaboration among scientists are essential if we are to do “the right thing” and offer children and families evidence of our unified hopes for their future.
THERAPEUTIC APPROACHES FOR PMM2-DEFICIENT PATIENTS
| Approach | Rationale | Advantages | Disadvantages |
|---|---|---|---|
| Enzyme Replacement | Provides normal active enzyme | Corrects defect in cells that take up and localize sufficient enzyme | Unequal accessibility to cells, especially CNS; would not cross blood brain barrier, requires cytoplasmic targeting; high cost |
| PMM2 Gene Therapy | Corrects defect by providing DNA encoding normal enzyme | Permanently corrects defect in cells that take up and integrate DNA | Variable accessibility to all cells, especially CNS, potential tumor formation, safety and regulatory hurdles, small number of patients |
| PMM2 Enzyme enhancement | Competitive inhibitor stabilizes mutated enzyme, but dissociates at lower pH to allow substrate binding | Small molecule may cross blood brain barrier; effective in some lysosomal storage disorders | pH does not change between site of synthesis and action; substrate competes with inhibitor |
| PMM2 activation | Non-competitive small molecule activates or stabilizes mutant enzyme increasing activity | Likely to cross BBB, may stabilize/activate in various ways | May not be useful for all mutant genotypes; will depend on whether specific mutation affects enzyme stability, Km, substrate binding or transcription |
| MPI Inhibition | Increase Man-6-P flux toward glycosylation by reducing MPI activity increaseing PMM2:MPI | Small molecule likely to cross BBB activity; addition of mannose to the diet rescues even severe MPI deficiency | May not be effective in all tissues; likely to benefit those with higher residual activity. |
| AICAR | Activate glycogen phosphorylase, driving glucose→ fructose-6-P and increase Man-6-P | Orally active drug mimics effects of insulin and mobilizes glycogen | Studies only done on cell containing 0.5mM glucose. Application to physiological conditions unknown |
Acknowledgments
This work was supported by National Institute of Diabetes, Digestive and Kidney Diseases (R01-DK55695), The Rocket Williams Fund, Sanford Children’s Health Research Center at the Burnham Institute for Medical Research, and the CDG Family Network Foundation and Mason’s Hope. We thank Dr. Vandana Sharma for her help with the figures in this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jaeken J, et al. About nomenclature of Congenital Disorders of Glycosylation (CDG) Journal of Inherited Metabolic Disease. 2008 doi: 10.1007/s10545-008-0983-x. In Press. [DOI] [PubMed] [Google Scholar]
- 2.Freeze HH. Genetic defects in the human glycome. Nat Rev Genet. 2006;7(7):537–51. doi: 10.1038/nrg1894. [DOI] [PubMed] [Google Scholar]
- 3.Grunewald S. Congenital disorders of glycosylation: rapidly enlarging group of (neuro)metabolic disorders. Early Hum Dev. 2007;83(12):825–30. doi: 10.1016/j.earlhumdev.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Jaeken J, Matthijs G. Congenital disorders of glycosylation: a rapidly expanding disease family. Annu Rev Genomics Hum Genet. 2007;8:261–78. doi: 10.1146/annurev.genom.8.080706.092327. [DOI] [PubMed] [Google Scholar]
- 5.Leroy JG. Congenital disorders of N-glycosylation including diseases associated with O- as well as N-glycosylation defects. Pediatr Res. 2006;60(6):643–56. doi: 10.1203/01.pdr.0000246802.57692.ea. [DOI] [PubMed] [Google Scholar]
- 6.Barone R, et al. Borderline mental development in a congenital disorder of glycosylation (CDG) type Ia patient with multisystemic involvement (intermediate phenotype) J Inherit Metab Dis. 2007;30(1):107. doi: 10.1007/s10545-006-0486-6. [DOI] [PubMed] [Google Scholar]
- 7.Briones P, et al. Biochemical and molecular studies in 26 Spanish patients with congenital disorder of glycosylation type Ia. J Inherit Metab Dis. 2002;25(8):635–46. doi: 10.1023/a:1022825113506. [DOI] [PubMed] [Google Scholar]
- 8.Coman D, et al. Congenital disorder of glycosylation type Ia in a 6-year-old girl with a mild intellectual phenotype: two novel PMM2 mutations. J Inherit Metab Dis. 2005;28(6):1189–90. doi: 10.1007/s10545-005-0166-y. [DOI] [PubMed] [Google Scholar]
- 9.Giurgea I, et al. Underdiagnosis of mild congenital disorders of glycosylation type Ia. Pediatr Neurol. 2005;32(2):121–3. doi: 10.1016/j.pediatrneurol.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Pancho C, et al. Congenital disorder of glycosylation type Ia revealed by hypertransaminasemia and failure to thrive in a young boy with normal neurodevelopment. J Pediatr Gastroenterol Nutr. 2005;40(2):230–2. doi: 10.1097/00005176-200502000-00030. [DOI] [PubMed] [Google Scholar]
- 11.Grünewald S, et al. High residual activity of PMM2 in patients’ fibroblasts: possible pitfall in the diagnosis of CDG-Ia (phosphomannomutase deficiency) Am J Hum Genet. 2001;68(2):347–54. doi: 10.1086/318199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westphal V, et al. A frequent mild mutation in ALG6 may exacerbate the clinical severity of patients with congenital disorder of glycosylation Ia (CDG-Ia) caused by phosphomannomutase deficiency. Hum Mol Genet. 2002;11(5):599–604. doi: 10.1093/hmg/11.5.599. [DOI] [PubMed] [Google Scholar]
- 13.Kepes F, Schekman R. The yeast SEC53 gene encodes phosphomannomutase. J Biol Chem. 1988;263(19):9155–61. [PubMed] [Google Scholar]
- 14.Thiel C, et al. Targeted disruption of the mouse phosphomannomutase 2 gene causes early embryonic lethality. Mol Cell Biol. 2006;26(15):5615–20. doi: 10.1128/MCB.02391-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pirard M, et al. Kinetic properties and tissular distribution of mammalian phosphomannomutase isozymes. Biochem J. 1999;339( Pt 1):201–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Cromphout K, et al. The normal phenotype of Pmm1-deficient mice suggests that Pmm1 is not essential for normal mouse development. Mol Cell Biol. 2006;26(15):5621–35. doi: 10.1128/MCB.02357-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veiga-da-Cunha M, et al. Mammalian phosphomannomutase PMM1 is the brain IMP-sensitive glucose-1,6-bisphosphatase. J Biol Chem. 2008;283(49):33988–93. doi: 10.1074/jbc.M805224200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahon RJ, Frost SC. Glycogen: a carbohydrate source for GLUT-1 glycosylation during glucose deprivation of 3T3-L1 adipocytes. Am J Physiol. 1996;270(4 Pt 1):E640–5. doi: 10.1152/ajpendo.1996.270.4.E640. [DOI] [PubMed] [Google Scholar]
- 19.Taguchi T, et al. Hepatic glycogen breakdown is implicated in the maintenance of plasma mannose concentration. Am J Physiol Endocrinol Metab. 2005;288(3):E534–40. doi: 10.1152/ajpendo.00451.2004. [DOI] [PubMed] [Google Scholar]
- 20.Korner C, Lehle L, von Figura K. Abnormal synthesis of mannose 1-phosphate derived carbohydrates in carbohydrate-deficient glycoprotein syndrome type I fibroblasts with phosphomannomutase deficiency. Glycobiology. 1998;8(2):165–71. doi: 10.1093/glycob/8.2.165. [DOI] [PubMed] [Google Scholar]
- 21.Körner C, Lehle L, von Figura K. Carbohydrate-deficient glycoprotein syndrome type 1: correction of the glycosylation defect by deprivation of glucose or supplementation of mannose. Glycoconj J. 1998;15(5):499–505. doi: 10.1023/a:1006939104442. [DOI] [PubMed] [Google Scholar]
- 22.Mavilio F, Ferrari G. Genetic modification of somatic stem cells. The progress, problems and prospects of a new therapeutic technology. EMBO Rep. 2008;9(Suppl 1):S64–9. doi: 10.1038/embor.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellor S, Shupe T, Petersen B. Stem cell therapy for inherited metabolic disorders of the liver. Exp Hematol. 2008;36(6):716–25. doi: 10.1016/j.exphem.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan AA, et al. Treatment of Crigler-Najjar Syndrome type 1 by hepatic progenitor cell transplantation: a simple procedure for management of hyperbilirubinemia. Transplant Proc. 2008;40(4):1148–50. doi: 10.1016/j.transproceed.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Aldenhoven M, Boelens JJ, de Koning TJ. The clinical outcome of Hurler syndrome after stem cell transplantation. Biol Blood Marrow Transplant. 2008;14(5):485–98. doi: 10.1016/j.bbmt.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Reismann P, Tulassay Z. Treatment prospects of lysosomal storage disorders. Orv Hetil. 2008;149(25):1171–9. doi: 10.1556/OH.2008.28382. [DOI] [PubMed] [Google Scholar]
- 27.Menniti M, et al. Serum and glucocorticoid-regulated kinase Sgk1 inhibits insulin-dependent activation of phosphomannomutase 2 in transfected COS-7 cells. Am J Physiol Cell Physiol. 2005;288(1):C148–55. doi: 10.1152/ajpcell.00284.2004. [DOI] [PubMed] [Google Scholar]
- 28.Niehues R, et al. Carbohydrate-deficient glycoprotein syndrome type Ib. Phosphomannose isomerase deficiency and mannose therapy. J Clin Invest. 1998;101(7):1414–20. doi: 10.1172/JCI2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harms HK, et al. Oral mannose therapy persistently corrects the severe clinical symptoms and biochemical abnormalities of phosphomannose isomerase deficiency. Acta Paediatr. 2002;91(10):1065–72. doi: 10.1080/080352502760311566. [DOI] [PubMed] [Google Scholar]
- 30.Kjaergaard S, et al. Failure of short-term mannose therapy of patients with carbohydrate-deficient glycoprotein syndrome type 1A. Acta Paediatr. 1998;87(8):884–8. doi: 10.1080/080352598750013680. [DOI] [PubMed] [Google Scholar]
- 31.Mayatepek E, Kohlmuller D. Mannose supplementation in carbohydrate-deficient glycoprotein syndrome type I and phosphomannomutase deficiency. Eur J Pediatr. 1998;157(7):605–6. doi: 10.1007/s004310050889. [DOI] [PubMed] [Google Scholar]
- 32.Panneerselvam K, Freeze HH. Mannose corrects altered N-glycosylation in carbohydrate-deficient glycoprotein syndrome fibroblasts. J Clin Invest. 1996;97(6):1478–87. doi: 10.1172/JCI118570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao N, Shang J, Lehrman MA. Analysis of glycosylation in CDG-Ia fibroblasts by fluorophore-assisted carbohydrate electrophoresis: implications for extracellular glucose and intracellular mannose 6-phosphate. J Biol Chem. 2005;280(18):17901–9. doi: 10.1074/jbc.M500510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehrman MA. Stimulation of N-linked glycosylation and lipid-linked oligosaccharide synthesis by stress responses in metazoan cells. Crit Rev Biochem Mol Biol. 2006;41(2):51–75. doi: 10.1080/10409230500542575. [DOI] [PubMed] [Google Scholar]
- 35.Rush JS, et al. Mannose supplementation corrects GDP-mannose deficiency in cultured fibroblasts from some patients with Congenital Disorders of Glycosylation (CDG) Glycobiology. 2000;10(8):829–35. doi: 10.1093/glycob/10.8.829. [DOI] [PubMed] [Google Scholar]
- 36.Higashidani A, et al. Exogenous Mannose Does Not Raise Steady State Mannose-6-Phosphate Pools of Normal or N-Glycosylation-Deficient Human Fibroblasts. Mol Genet Metabol. 2009 doi: 10.1016/j.ymgme.2008.12.005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeRossi C, et al. Ablation of mouse phosphomannose isomerase (Mpi) causes mannose 6-phosphate accumulation, toxicity, and embryonic lethality. J Biol Chem. 2006;281(9):5916–27. doi: 10.1074/jbc.M511982200. [DOI] [PubMed] [Google Scholar]
- 38.Eklund EA, et al. Hydrophobic Man-1-P derivatives correct abnormal glycosylation in Type I congenital disorder of glycosylation fibroblasts. Glycobiology. 2005;15(11):1084–93. doi: 10.1093/glycob/cwj006. [DOI] [PubMed] [Google Scholar]
- 39.Rutschow S, et al. Membrane-permeant derivatives of mannose-1-phosphate. Bioorg Med Chem. 2002;10(12):4043–9. doi: 10.1016/s0968-0896(02)00269-9. [DOI] [PubMed] [Google Scholar]
- 40.Hardre R, et al. Mono, di and tri-mannopyranosyl phosphates as mannose-1-phosphate prodrugs for potential CDG-Ia therapy. Bioorg Med Chem Lett. 2007;17(1):152–5. doi: 10.1016/j.bmcl.2006.09.074. [DOI] [PubMed] [Google Scholar]
- 41.Rawat M, et al. Lipid carriers: a versatile delivery vehicle for proteins and peptides. Yakugaku Zasshi. 2008;128(2):269–80. doi: 10.1248/yakushi.128.269. [DOI] [PubMed] [Google Scholar]
- 42.Beck M. New therapeutic options for lysosomal storage disorders: enzyme replacement, small molecules and gene therapy. Hum Genet. 2007;121(1):1–22. doi: 10.1007/s00439-006-0280-4. [DOI] [PubMed] [Google Scholar]
- 43.Desnick RJ. Enzyme replacement and enhancement therapies for lysosomal diseases. J Inherit Metab Dis. 2004;27(3):385–410. doi: 10.1023/B:BOLI.0000031101.12838.c6. [DOI] [PubMed] [Google Scholar]
- 44.Pierret C, Morrison JA, Kirk MD. Treatment of lysosomal storage disorders: focus on the neuronal ceroid-lipofuscinoses. Acta Neurobiol Exp (Wars) 2008;68(3):429–42. doi: 10.55782/ane-2008-1709. [DOI] [PubMed] [Google Scholar]
- 45.Snyder EL, Dowdy SF. Cell penetrating peptides in drug delivery. Pharm Res. 2004;21(3):389–93. doi: 10.1023/B:PHAM.0000019289.61978.f5. [DOI] [PubMed] [Google Scholar]
- 46.Schwarze SR, et al. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285(5433):1569–72. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 47.Elson-Schwab L, et al. Guanidinylated neomycin delivers large, bioactive cargo into cells through a heparan sulfate-dependent pathway. J Biol Chem. 2007;282(18):13585–91. doi: 10.1074/jbc.M700463200. [DOI] [PubMed] [Google Scholar]
- 48.Boini KM, et al. Role of serum- and glucocorticoid-inducible kinase SGK1 in glucocorticoid regulation of renal electrolyte excretion and blood pressure. Kidney Blood Press Res. 2008;31(4):280–9. doi: 10.1159/000151666. [DOI] [PubMed] [Google Scholar]
- 49.Silvaggi NR, et al. The X-ray crystal structures of human alpha-phosphomannomutase 1 reveal the structural basis of congenital disorder of glycosylation type 1a. J Biol Chem. 2006;281(21):14918–26. doi: 10.1074/jbc.M601505200. [DOI] [PubMed] [Google Scholar]
- 50.Mu TW, et al. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell. 2008;134(5):769–81. doi: 10.1016/j.cell.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shang J, Lehrman MA. Metformin-stimulated mannose transport in dermal fibroblasts. J Biol Chem. 2004;279(11):9703–12. doi: 10.1074/jbc.M310837200. [DOI] [PubMed] [Google Scholar]
- 52.Lochhead PA, et al. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes. 2000;49(6):896–903. doi: 10.2337/diabetes.49.6.896. [DOI] [PubMed] [Google Scholar]
- 53.Shang J, Lehrman MA. Activation of glycogen phosphorylase with 5-aminoimidazole-4-carboxamide riboside (AICAR). Assessment of glycogen as a precursor of mannosyl residues in glycoconjugates. J Biol Chem. 2004;279(13):12076–80. doi: 10.1074/jbc.M400431200. [DOI] [PubMed] [Google Scholar]
- 54.Narkar VA, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134(3):405–15. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gracy RW, Noltmann EA. Studies on phosphomannose isomerase. II. Characterization as a zinc metalloenzyme. J Biol Chem. 1968;243(15):4109–16. [PubMed] [Google Scholar]
- 56.Noltman E. In: The Enzymes. Boyer PD, editor. Vol. 6. 1972. pp. 271–354. [Google Scholar]
- 57.Jaeken J, et al. Inhibition of phosphomannose isomerase by fructose 1-phosphate: an explanation for defective N-glycosylation in hereditary fructose intolerance. Pediatr Res. 1996;40(5):764–6. doi: 10.1203/00006450-199611000-00017. [DOI] [PubMed] [Google Scholar]
- 58.Bhandari A, et al. Exploring structure-activity relationships around the phosphomannose isomerase inhibitor AF14049 via combinatorial synthesis. Bioorg Med Chem Lett. 1998;8(17):2303–8. doi: 10.1016/s0960-894x(98)00417-x. [DOI] [PubMed] [Google Scholar]
- 59.Cleasby A, et al. The x-ray crystal structure of phosphomannose isomerase from Candida albicans at 1.7 angstrom resolution. Nat Struct Biol. 1996;3(5):470–9. doi: 10.1038/nsb0596-470. [DOI] [PubMed] [Google Scholar]
- 60.Huryn DM, Cosford N. The Molecular Libraries Screening Center Network (MLSCN): Identifying Chemical Probes of Biological Systems. Annual Reports in Medicinal Chemistry. 2007;42(Chapter 26):401. doi: 10.1016/S0065-7743(07)42026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis JA, Freeze HH. Studies of mannose metabolism and effects of long-term mannose ingestion in the mouse. Biochim Biophys Acta. 2001;1528(2–3):116–26. doi: 10.1016/s0304-4165(01)00183-0. [DOI] [PubMed] [Google Scholar]
- 62.Alton G, et al. Direct utilization of mannose for mammalian glycoprotein biosynthesis. Glycobiology. 1998;8(3):285–95. doi: 10.1093/glycob/8.3.285. [DOI] [PubMed] [Google Scholar]
- 63.Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006;1(1):241–5. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- 64.Ho SY, et al. Zebrafish fat-free is required for intestinal lipid absorption and Golgi apparatus structure. Cell Metab. 2006;3(4):289–300. doi: 10.1016/j.cmet.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thornhill P, et al. Developmental defects in a zebrafish model for muscular dystrophies associated with the loss of fukutin-related protein (FKRP) Brain. 2008;131(Pt 6):1551–61. doi: 10.1093/brain/awn078. [DOI] [PubMed] [Google Scholar]
- 66.Shang J, et al. Translation attenuation by PERK balances ER glycoprotein synthesis with lipid-linked oligosaccharide flux. J Cell Biol. 2007;176(5):605–16. doi: 10.1083/jcb.200607007. [DOI] [PMC free article] [PubMed] [Google Scholar]