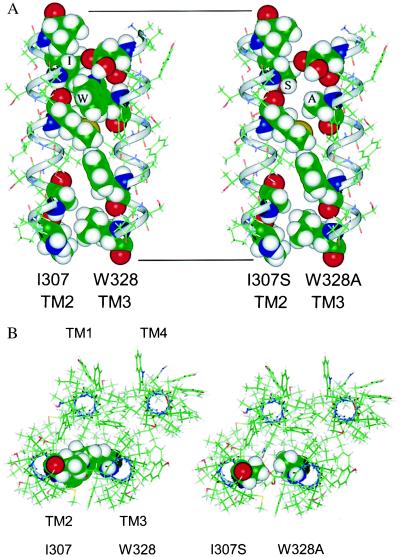
Figure 4.
(A) Molecular model of TM2 and TM3 of the human GABA ρ1 receptor subunit. The wild-type TM domains are shown on the left; the I307S/W328A double mutant TM domains are shown on the right. The predicted TM2 was modeled as an α-R-helix. However, in the assignment of the TM2 residues, those proposed by Unwin (22) for the nearly homologous TM2 of the nicotinic acetylcholine receptor (Val-308 to Val-325 of the GABA-R-ρ1) were used rather than residues Ala-303 to Val-325 of the GABA ρ1 receptor proposed by Cutting et al. (23). The side of TM2 lining the ion channel pore is on the far left. TM3 was also modeled as an α-helix. The axis of TM3 was aligned antiparallel with that of TM2 and then TM3 was rotated about its axis to obtain the closest approach of Ile-307 to Trp-328. Finally, the nonbonded interactions between the two helices were optimized. As shown on the right, the double mutation of the GABA ρ1 receptor potentially creates a substantial cavity between the TM2 and TM3 α-helices. (B) A model of a GABA-R-ρ1 subunit viewed along the axis of the ion channel from the extracellular side. The dimer of TM2 and TM3 shown in A was used as a starting point for a model of a tetrameric subunit. TM2 was positioned so that the polar residues that line the ion channel face to the left. TM1 was built as an α-helix (23) and aligned antiparallel with TM2 so that its axis was 11 Å from both TM2 and TM3 (an equilateral triangle). TM4 was built as an α-helix and aligned antiparallel to TM3 so that its axis was 11 Å from TM3 and TM1. The backbone atoms of the four helices were tethered with a force constant of 10 kcal/Å (1 cal = 4.184 J). The positions of TM1 and TM4 were based on the data of Blanton and Cohen (24), demonstrating extent of exposure of the TMs of the acetylcholine receptor to hydrophobic photoactivatable probes: TM1 was only slightly labeled, TM3 was partially labeled, and TM4 was most labeled. The subunit on the left is wild-type GABA-R-ρ1 that has residues Ile-307 and Trp-328 shown with a van der Waals surface. The subunit on the right has the I307S and W328A mutations shown with a van der Waals surface.