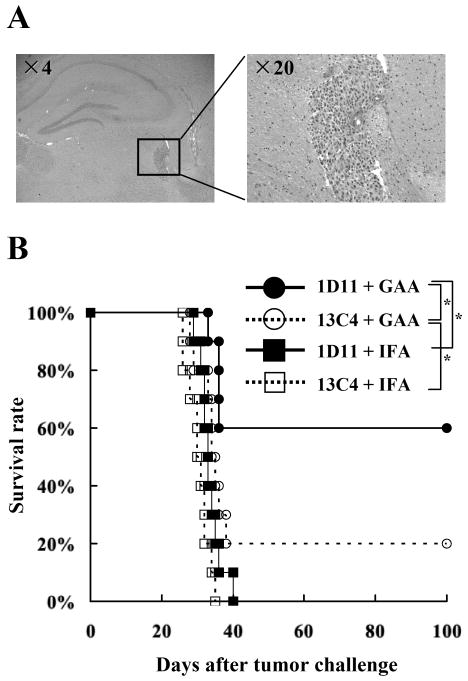
Figure 1. Systemic inhibition of TGF-β improves the therapeutic efficacy of vaccinations targeting GAA-derived CTL epitopes.
On day 0, each C57BL/6 mouse received a stereotactic injection with 1×105 GL261 glioma cells into the right basal ganglia. Starting on day 3, anti–TGF-β antibody (1D11) or control antibody (13C4) were administered i.p. at a dose of 25mg/kg every two days for a total of twelve doses. On days 3, 13, and 23, mice received s.c. immunization with 100 μg of each GAA peptide and HBVcore128 T-helper epitope peptide emulsified in IFA. A, H&E staining of frozen brain sections derived from C57BL/6 mice bearing day 3 GL261 glioma in the right basal ganglia. B, the mice were stratified into 4 treatment groups: 1) 1D11 plus GAA-vaccines (solid circles); 2) 13C4 plus GAA-vaccines (hollow circles); 3) 1D11 plus control vaccines with HBVcore128 T-helper epitope peptide emulsified in IFA alone (solid squares) and 4) 13C4 plus control vaccines (hollow squares). Symptom-free survival of mice was monitored. n = 10 mice/group. *, p < 0.05 for the mice treated with 1D11 plus GAA-vaccines compared with the mice treated with 13C4 plus GAA-vaccines or 1D11 plus control vaccines *, p < 0.05 for the mice treated with 13C4 plus GAA-vaccines compared with the mice treated with 13C4 plus control vaccines.