Figure 1. Glial activation by peripheral neuropathy.
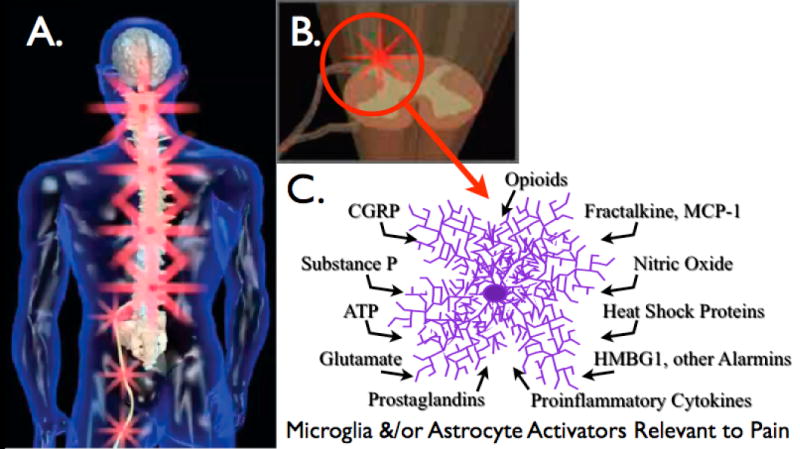
(A) Peripheral nerve injury leads to spinal amplification of incoming pain messages in addition to spontaneous pain signaling. This is indicated by the magnification of red “stars” symbolizing pain signals moving from the periphery into spinal cord, with amplification of pain messages being relayed to higher brain centers. (B) Spinal amplification of pain occurs within the spinal cord dorsal horn, a key site for the dynamic regulation of pain processing. This is the site where incoming sensory fibers synapse with neurons that relay pain messages up to brain via the spinothalamic and other pathways. This is also the site where glia and other immunocompetent cells can amplify pain via the release of neuroexcitatory substances such as proinflammatory cytokines. (C) The release of neuroexcitory, proinflammatory products by glia occurs in response to microglial and astrocyte activation. This activated state can occur in response to a variety of neuron-to-glia signals including neuronal chemokines (fractalkine, MCP-1), neurotransmitters (glutamate, ATP, substance P, CGRP), neuromodulators (nitric oxide, prostaglandins), endogenous danger signals (also called “alarmins”; e.g., heat shock proteins, the nuclear protein HMGB1), in addition to xenobiotics including opioids.
