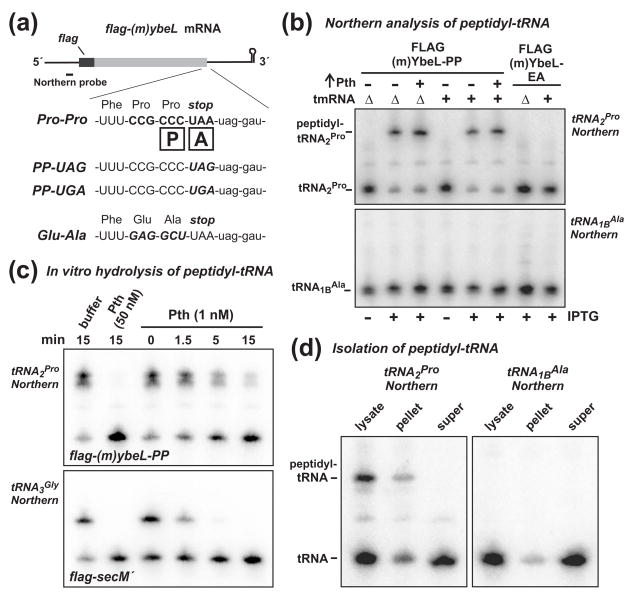
Figure 1. Peptidyl prolyl-tRNA2Pro accumulates during inefficient translation termination.
(a) FLAG-(m)YbeL expression constructs. The flag-(m)ybeL message is shown schematically along with the Northern probe binding site. The 3′ coding sequences of flag-(m)ybeL-PP, -PP(uag), -PP(uga), and -EA are shown. The boxed P and A indicate the positions of the ribosomal P and A sites during translation termination. (b) Northern blot analysis of peptidyl-tRNA. Total RNA was isolated from tmRNA+ and ΔtmRNA cells, resolved on acid-urea polyacrylamide gels, and analyzed by Northern blot hybridization using probes specific for tRNA2Pro and tRNA1BAla. Expression of FLAG-(m)YbeL-PP (+ IPTG) resulted in the accumulation of peptidyl prolyl-tRNA2Pro in both tmRNA+ and ΔtmRNA cells, whereas no peptidyl-tRNA was detected in cells expressing FLAG-(m)YbeL-EA. Overexpression of Pth had no significant effect on peptidyl prolyl-tRNA2Pro levels. (c) Treatment of peptidyl-tRNA with Pth-His6 in vitro. Total RNA from cells expressing FLAG-(m)YbeL-PP or FLAG-SecM′ was treated with purified Pth-His6 for the indicated time, and analyzed by Northern blot hybridization for tRNA2Pro or tRNA3Gly, respectively. (d) Fractionation of peptidyl-tRNA. Cells expressing FLAG-(m)YbeL-PP were broken by French press and fractionated by sucrose density ultracentrifugation. RNA was extracted from the lysate, pellet, and supernatant fractions, and analyzed by Northern blot for tRNA2Pro and tRNA1BAla. Peptidyl prolyl-tRNA2Pro was only found in the high-speed pellet fraction.