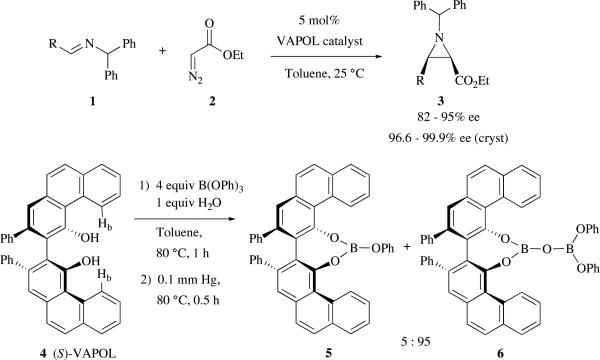
Investigations over the last decade have inveterated the privileged nature of VAPOL (and VANOL) as a ligand in the catalytic asymmetric aziridination reaction (AZ reaction) of imines with diazo compounds.1,2 With ethyl diazoacetate, ethyl aziridine-2-carboxylates are obtained with high cis-selectivity and enantioselectivity from benzhydryl imines derived from a range of aldehydes including 1°, 2° and 3° aliphatic aldehydes and from a range of electron rich and electron poor aryl aldehydes (Scheme 1).1g More recently, we reported that even higher asymmetric inductions could be obtained with certain substituted benzhydryl imine derivatives.1h Our studies have revealed that the procedure for catalyst formation generates a mixture of two species which we tentatively identified as mesoborate 5 and pyroborate 6 (Scheme 1).1g The present work suggests that the active catalyst is not a Lewis acid of the type 5 or 6 but in fact a Brønsted acid of singular structure.
Scheme 1.
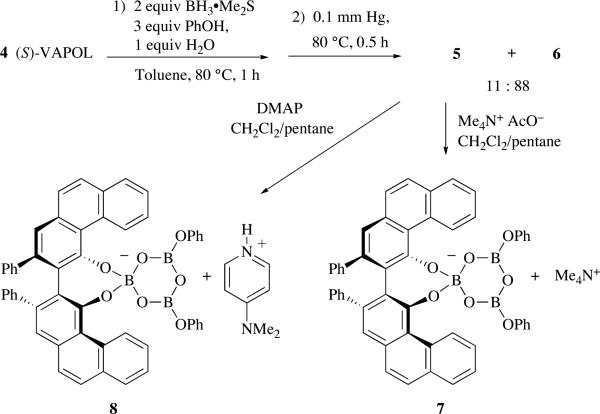
The structural assignment of pyroborate 6 was based on its 1H NMR and 11B NMR spectra and on its high resolution mass spectrum.1g Efforts to obtain solid state structural support for the assignment of pyroborate 6 were thwarted by the fact that 6 is not crystalline. In this regard, we were drawn to a report by Aldridge and coworkers who were able to structurally characterize a bis-Lewis base adduct of a simple pyroborate and acetate ion.3 In the procedure for the generation of 6 shown in Scheme 1, the excess B(OPh)3 is not removed under high vacuum. In an effort to generate a cleaner sample of pyroborate 6, the procedure outlined in Scheme 2 was developed with the more volatile BH3•Me2S which resulted in a 11:88 mixture of mesoborate 5 to pyroborate 6 with ≤10% of unreacted VAPOL remaining. Subsequent treatment of this mixture with one equivalent of tetramethylammonium acetate followed by crystallization from CH2Cl2 and pentane gave crystals of diffraction quality. X-ray diffraction analysis of these crystals revealed that the compound produced from this reaction was not the bis-Lewis base adduct of pyroborate 6 and acetate ion. Quite remarkably, these crystals proved to be the ion pair 7 consisting of the tetramethylammonium cation and an anion formed from a boroxine ring in which one of the three borons forms a spiroborate anion (boroxinate) with a molecule of VAPOL.
Scheme 2.
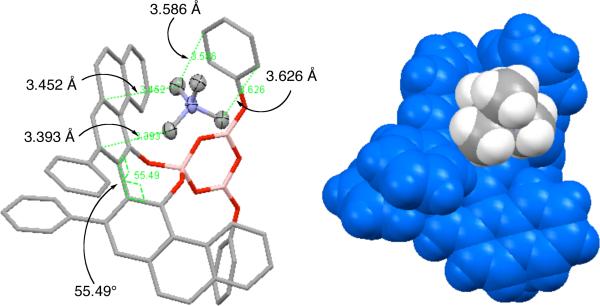
The crystal structure of 7 reveals that three of the methyl groups in the tetramethylammonium ion have close contacts with the aromatic rings of the boroxinate anion (Figure 1). These contacts are less than the sum of the van der Waals radii of an sp3 carbon (2.0 Å) and an sp2 carbon (1.7 Å) and are reminiscent of the cation-π interactions described by Dougherty and Stauffer.4,5 These interactions occur with one of the phenanthrene rings of the VAPOL ligand and also with one of the phenyloxy rings of the boroxinate core which folds up to meet the tetramethylammonium cation. The twist angle about the biaryl axis is 55° in boroxinate 7 which is to be compared with 79° in the free VAPOL ligand.6
Figure 1.
Crystal structure of the boroxinate ammonium salt 7 visualized by the Mercury Program
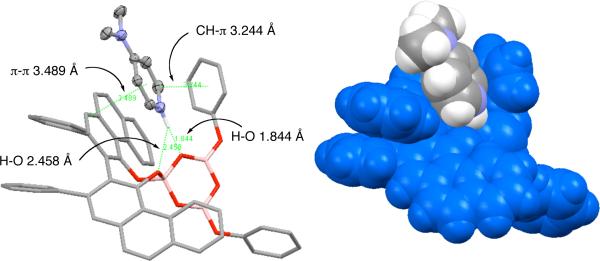
The redistribution of the pyroborate 6 into the boroxinate 7 mediated by acetate ion was quite unexpected. In an effort to determine if this was a function of the bidentate nature of the acetate ion, an attempt was made to trap the pyroborate 6 with a mono-dentate Lewis base, 4-dimethylamino pyridine (DMAP) that we hoped would mimic the imine substrates in the AZ reaction. Treatment of the 11:88 mixture of 5 and 6 generated as shown in Scheme 2 with 1 equivalent of DMAP gave the DMAP complex 8 which crystallized from solution in 23% yield. As indicated in Figure 2, this compound also has a boroxinate core that is complexed with a protonated DMAP. The key interaction in this complex is the hydrogen bond between the protonated nitrogen and one of the oxygens of the boroxine ring that is attached to the 4-coordinate boron in which the N-O distance is 2.850 Å. The hydrogen was initially located closer to the nitrogen than to the oxygen and further refinement was consistent with proton transfer to the nitrogen with an N-H bond of 1.006 Å. The hydrogen bond also appears to be bifurcated7,8 with an additional interaction with one of the oxygens on the VAPOL ligand. The H-Oboroxine distance is 1.844 Å and the H-OVAPOL distance is 2.458 Å.9 There also appears to be a π-π stacking interaction10 (3.489 Å) between a phenanthrene ring of the VAPOL ligand and the pyridinium ion of the protonated DMAP. The two ring systems are 6.5° from parallel. Finally, there appears to be a CH-π interaction5 (3.244 Å) between an ortho-hydrogen on the DMAP ring and one of the phenoxy rings of the boroxine system. It is interesting to note that this phenoxy ring seems to have folded to meet the DMAP unit whereas the other phenoxy ring is projected out away from the boroxinate complex (Figure 2).
Figure 2.
Crystal structure of boroxinate-pyridinium complex 8 visualized by the Mercury Program
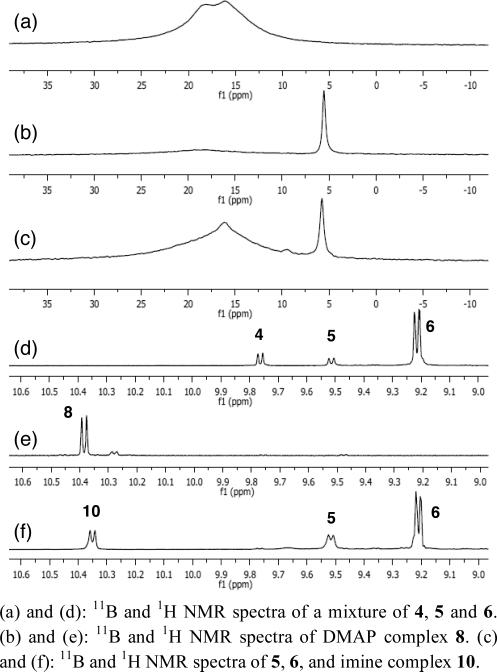
The 11B and 1H NMR spectra of the DMAP-boroxinate complex 8 are quite distinctive (Figure 3, spectra b and e). Three coordinate borate esters typically have broad absorptions for the boron between 16-20 ppm in CDCl3. The 11B NMR spectrum of B(OPh)3 reveals an absorption at 16.5 ppm. The unsymmetrical pyroborate 6 has two broad absorptions at 18.3 and 16.2 ppm in CDCl3 and for comparison this spectrum is shown in Figure 3 (spectrum a).1g Since 11B is a quadrapole, the sharpness of the absorption is related to the spherical symmetry around the boron and this is reflected in the appearance of the 11B NMR spectrum of the DMAP complex 8 (Figure 3, spectrum b) The two three coordinate borons in 8 appear as a very broad absorption at 19.31 ppm and the four coordinate boron as a very sharp peak at 5.54 ppm with an integration of 2:1, respectively (not shown). An upfield shift for the four coordinate boron is expected as a result of the increased electron density around the boron in the borate anion. For example, the 11B NMR absorption for LiB(OPh)4 is at 3.0 ppm.11 We are not aware of any boroxinate structures with which to compare the 11B shifts in the DMAP complex 8, but an adduct of piperidine and trimethylboroxine has been reported to have two signals in CD2Cl2 at 32.4 and 6.0 ppm.12
Figure 3.
(a) and (d): 11B and 1H NMR spectra of a mixture of 4, 5 and 6. (b) and (e): 11B and 1H NMR spectra of DMAP complex 8. (c) and (f): 11B and 1H NMR spectra of 5, 6, and imine complex 10.
The most distinctive absorption for the DMAP complex 8 in the 1H NMR spectrum is the bay-region doublet at 10.38 ppm (Figure 3, spectrum e). The bay proton of VAPOL (Hb in 4 in Scheme 1) is a very useful spectroscopic handle since it is shifted downfield by ~2 ppm from all other protons in the VAPOL ligand. The bay proton for free VAPOL is found at 9.77 ppm and that for the VAPOL mesoborate 5 at 9.51 ppm and the pyroborate 6 at 9.22 ppm (Figure 3, spectrum d).
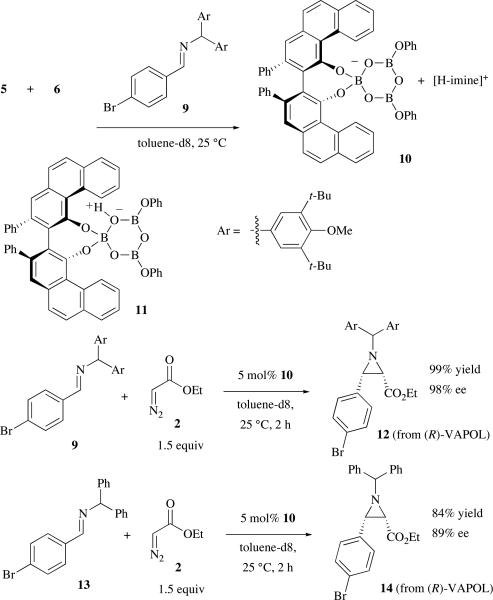
We have recently shown that tetrabutyldianisylmethyl (BUDAM) imines of the type 9 (Scheme 3) will react with ethyl diazoacetate in the presence of the VAPOL-B(OPh)3 catalyst to gives aziridines with exceptionally high asymmetric inductions.1h Treatment of the mixture of 5 and 6 prepared as indicated in Scheme 2 with 1 equivalent of the imine 9 lead to the formation of a complex that did not form single crystals. This complex has been tentatively assigned as the ion pair 10 consisting of the VAPOL boroxinate anion and the protonated form of imine 9. This assignment is made on the basis of its 11B and 1H NMR spectra, part of which are shown in Figure 3 (spectra c and f). The 11B NMR spectrum reveals a sharp peak for 10 at 5.7 ppm similar to that observed for the DMAP complex 8 (5.5 ppm). In addition, a bay region doublet for 10 is observed at 10.35 ppm which is also similar to that observed for the DMAP complex 8 (10.38 ppm). The proton of the protonated DAMP in 8 is found at 12.32 ppm and that for the protonated imine 9 in 10 is found at 13.65 ppm.
Scheme 3.
The initial preparation of the mixture of 5 and 6 from VAPOL and 2 equivalents of BH3•Me2S (Scheme 2) was developed with the intention of optimizing the formation of the pyroborate 6. This stoichiometry does not provide enough boron for the formation of the boroxinate 10 and this is presumably the reason that the 1H NMR spectrum of 10 reveals the substantial presence of the mesoborate 5 (9.51 ppm) and the pyroborate 6 (9.22 ppm) (Figure 3, spectrum f). However, if 10 is prepared from VAPOL, 4 equiv B(OPh)3, 1 equiv H2O and then 3 equiv of imine 9, a clean solution of 10 is generated containing 1% VAPOL, <1% of 5, and 5% of 6 (see Supporting Information). The viability of the species 10 to function as a catalyst for the AZ reaction was then examined. Treatment of 9 and EDA with 5 mol% of 10 gave the aziridine 12 in 99% yield and 98% ee which compares with the reported data for this reaction in the AZ reaction (98% yield and 99% ee).1h,13 The ion pair 10 generated from the imine 9 will also catalyze the reaction of an imine with a different N-substituent. The aziridine 14 was produced in 84% yield and 89% ee and we have previously observed that this aziridine can be obtained from imine 13 in 78% yield and 90% ee with catalysts generated from B(OPh)3.1g,13
The original optimized procedure1g for the preparation of the aziridination catalyst involved reacting VAPOL with three equivalents of B(OPh)3 and this is consistent with a boroxinate as the active catalyst. The same 1:3 stoichiometry was also optimal in a catalyst for an asymmetric heteroatom Diels-Alder reaction.14 Neither procedure however involved the addition of any H2O. The formation of a boroxine requires 3 equivalents of H2O. The success and reproducibility of the AZ reaction apparently has depended on the fact that commercial B(OPh)3 is never pure and contains partially hydrolyzed boron containing compounds.
Thus if the boroxinate is the active catalyst in the AZ reaction, then after the aziridine is liberated, the resulting species would be the protonated boroxinate 11 although we have not been able to detect this species. Treatment of 10 with 1 equivalent of EDA results in the quantitative formation of 12 and to the loss of all absorptions below 10 ppm in the 11B NMR (see supporting information). Ostensibly, 11 could be either a chiral Lewis acid or a chiral Brønsted acid but given the structure of the DMAP boroxinate complex 8 and the similarities of the 1H and 11B NMR spectra of 8 and 10, it is considered likely that the imine 9 is bound in 10 in a protonated form and that the AZ reaction involves a chiral Brønsted acid and not a chiral Lewis acid as had been assumed.15 The implications of the discovery of this new class of chiral Brønsted acids will be reported in due course.
Supplementary Material
Acknowledgment
This work was supported by NIH Grant GM 63019. We thank Daniel Holmes for assistance with NMR experiments.
Footnotes
Supporting Information Available: Synthetic procedures and spectral data for all new compounds and X-ray diffraction data and cif files for 7 and 8. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.For catalytic asymmetric aziridinations of imines and diazo compounds with VANOL and VAPOL ligands, see: Antilla JC, Wulff WD. J. Am. Chem. Soc. 1999;121:5099–5100.Antilla JC, Wulff WD. Angew. Chem. Int. Ed. 2000;39:4518–4521.Loncaric C, Wulff WD. Org. Lett. 2001;3:3675–3678. doi: 10.1021/ol010180x.Patwardan A, Pulgam VR, Zhang Y, Wulff WD. Angew. Chem. Int. Ed. 2005;44:6169–6172. doi: 10.1002/anie.200500923.Deng Y, Lee YR, Newman CA, Wulff WD. Eur. J. Org. Chem. 2007:2068–2071.Lu Z, Zhang Y, Wulff WD. J. Am. Chem. Soc. 2007;129:7185–7194. doi: 10.1021/ja069371r.Zhang Y, Desai A, Lu Z, Hu G, Ding Z, Wulff WD. Chem. Eur. J. 2008;14:3785–3803. doi: 10.1002/chem.200701558.Zhang Y, Lu Z, Desai A, Wulff WD. Org. Lett. 2008;10:5429–5432. doi: 10.1021/ol802431v.
- 2.For catalytic asymmetric aziridinations of imines and diazo compounds with other ligands, see: Rasumussen KG, Jorgensen KA. J. Chem. Soc. Perkin Trans 1. 1997:1287.Juhl K, Hazell RG, Jorgensen KA. J. Chem. Soc. Perkin Trans 1. 1999:2293.Mayer MF, Hossain MM. J. Organometallic Chem. 2002;654:202.Krumper JR, Gerisch M, Suh JM, Bergman RG, Tilley TD. J. Org. Chem. 2003;68:9705. doi: 10.1021/jo035120e.Redlich M, Hossain MM. Tetrahedron Lett. 2004;45:8987.Wipf P, Lyon AM. ARKIVOC. 2007;xii:91.Hashimoto T, Uchiyama N, Maruoka K. J. Am. Chem. Soc. 2008;130:14380. doi: 10.1021/ja805635c.
- 3.Coombs ND, Aldridge S, Wiltshire G, Kays (nee Coombs) DL, Bresner C, Ooi L-L. J. Organometal. Chem. 2005;690:2725–2731. [Google Scholar]
- 4.Dougherty DA, Stauffer D. Science. 1990;250:1558. doi: 10.1126/science.2274786. [DOI] [PubMed] [Google Scholar]
- 5.For reviews, see: Nishio M. Tetrahedron. 2005;61:6923–6950.Nishio M, Hirota M, Umezawa Y. The CH/π Interaction. Wiley-VCH; 1998.
- 6.Price CP, Matzger AJ. J. Org. Chem. 2005;70:1. doi: 10.1021/jo048853n. [DOI] [PubMed] [Google Scholar]
- 7.Jeffrey GA. An Introduction to Hydrogen Bonding. Oxford University Press; 1997. [Google Scholar]
- 8.a Rozas I, Alkorta I, Elguero J. J. Phys. Chem. A. 1998;102:9925–9932. [Google Scholar]; b Bertolasi V, Gilli P, Ferretti V, Vaughan K. New. J. Chem. 1999;23:1261–1267. [Google Scholar]
- 9.See page 66 in reference 7 for a discussion.
- 10.For recent reviews, see: Janiak C. J. Chem. Soc., Dalton Trans. 2000:3885–3896.Roesky HW, Andruh M. Coord. Chem. Rev. 2003;236:91–119.Bhosale S, Sisson A, Sakai N, Matile S. Org. Biomol. Chem. 2006;4:3031–3039. doi: 10.1039/b606487f.
- 11.Ishiharta K, Kuirhara H, Matsumoto M, Yamamoto H. J. Am. Chem. Soc. 1998;120:6920. [Google Scholar]
- 12.Beckett MA, Brassington DS, Owen P, Hursthouse MB, Light ME, Malik KMA, Varma KS. J. Organomet. Chem. 1999;585:7. [Google Scholar]
- 13.The reaction times reported for 121h and 141g is 24 h, however, the minimum reaction time was not determined.
- 14.Newman CA, Antilla JC, Chen P, Predeus AV, Fielding L, Wulff WD. J. Am. Chem. Soc. 2007;129:7216. doi: 10.1021/ja069019d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brønsted acids are known to catalyze the aziridination of imines with diazo compounds, see reference 2g and: Williams A, Johnston JN. J. Am. Chem. Soc. 2004;126:1612. doi: 10.1021/ja0385282.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.