Table 2.
Reactions with Sulfur Electrophilesa
 | ||||
|---|---|---|---|---|
| reagent | ||||
| entry | arene | base | product | yield |
| 1 | 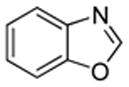 |
S tBuOLi |
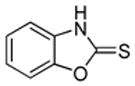 |
90% |
| 2 | 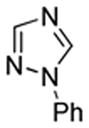 |
S tBuOLi |
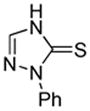 |
74% |
| 3 | 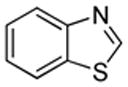 |
PhSSPh K3PO4 |
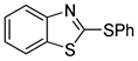 |
85% |
| 4 | 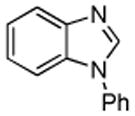 |
PhSSPh tBuOLi |
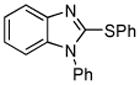 |
84% |
| 5 | 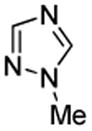 |
PhSSPh tBuOLi |
 |
55% |
| 6 | 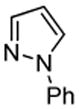 |
PhSSPh tBuOK |
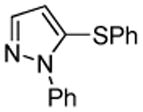 |
81% |
| 7 | 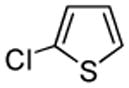 |
PhSSPh tBuOLi |
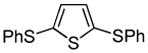 |
82% |
Substrate (1 equiv), base (1.5–3.0 equiv), sulfur (8 equiv) or PhSSPh (1.5–2.0 equiv). Yields are isolated yields. See the Supporting Information for details.
