Figure 1.
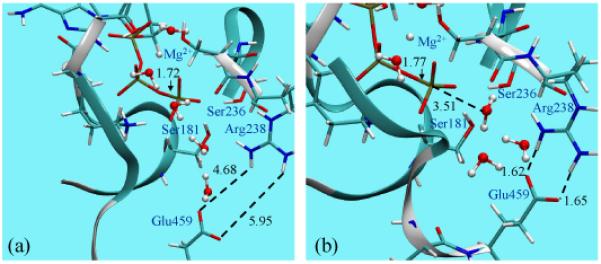
Comparison of the optimized active site of myosin motor domain with ATP bound to the (a) open (post-rigor) and (b) closed (pre-powerstroke) conformation state. The geometries have been optimized at the B3LYP/6-31G(d)/MM level (see text). Note the significant difference between the optimized Pγ-O3β bond distance in ATP; for reference, the equilibrium bond distance is ~1.69-1.70 Å in solution56,57.
