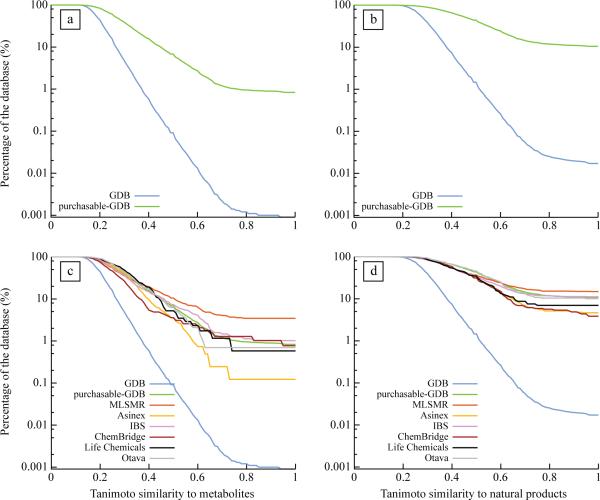
Figure 2. Compounds in screening libraries are biased toward biogenic molecules.
Percentage of the GDB and purchasable-GDB databases as a function of the Tanimoto similarity to their nearest neighbor in [a] the KEGG and [b] the Dictionary of Natural Compound databases. Percentage of the GDB, the purchasable-GDB, Asinex (360 042 compounds − 815 GDB compliant compounds), Chembridge (473 745 compounds − 389 GDB compliant compounds); IBS (424 806 compounds − 884 GDB compliant compounds), Life Chemicals (285 581 compounds − 172 GDB compliant compounds), Otava (121 657 compounds − 287 GDB compliant compounds) databases as a function of the Tanimoto similarity to their nearest neighbor to the [c] KEGG and the [d] Dictionary of Natural Products databases.