Abstract
Repetitive motion disorders, such as carpal tunnel syndrome and focal hand dystonia, can be associated with tasks that require prolonged, repetitive behaviors. Previous studies using animal models of repetitive motion have correlated cortical neuroplastic changes or peripheral tissue inflammation with fine motor performance. However, the possibility that both peripheral and central mechanisms coexist with altered motor performance has not been studied. In this study, we investigated the relationship between motor behaviour changes associated with repetitive behaviors and both peripheral tissue inflammation and cortical neuroplasticity. A rat model of reaching and grasping involving moderate repetitive reaching with negligible force (MRNF) was used. Rats performed the MRNF task for 2 hrs/day, 3 days/wk for 8 weeks. Reach performance was monitored by measuring reach rate/success, daily exposure, reach movement reversals/patterns, reach/grasp phase times, grip strength and grooming function. With cumulative task exposure, reach performance, grip strength and agility declined while an inefficient food retrieval pattern increased. In S1 of MRNF rats, a dramatic disorganization of the topographic forepaw representation was observed, including the emergence of large receptive fields located on both the wrist/forearm and forepaw with alterations of neuronal properties. In M1, there was a drastic enlargement of the overall forepaw map area, and of the cortex devoted to digit, arm-digits and elbow-wrist responses. In addition, unusually low current amplitude evoked digit movements. IL-1β and TNF-α increased in forearm flexor muscles and tendons of MRNF animals. The increases in IL-1β and TNF-α negatively correlated with grip strength and amount of current needed to evoke forelimb movements. This study provides strong evidence that both peripheral inflammation and cortical neuroplasticity jointly contribute to the development of chronic repetitive motion disorders.
Keywords: Repetitive motion injury, movement disorders, primary somatosensory cortex, primary motor cortex, electrophysiological mapping, inflammation, muscle, tendon, TNFalpha, IL-1beta
Introduction
Repetitive motion injuries affecting hands and digits can result from repeated grasping, typing or playing a musical instrument. Carpal tunnel syndrome is one common example of a repetitive motion disorder. Patients with this nerve entrapment syndrome have pain, paresthesias, numbness and weakness in the hand and wrist that may travel into the forearm, elbow, and shoulder. Usually this injury is associated with performance of repetitive tasks (Clark et al., 2003, 2004; Sommerich et al., 2007; Elliott et al., 2009a).
Maladaptive movement pattern changes may also be associated with chronic repetitive opening and closing hand tasks, with or without force (Byl et al., 1996, 1997; Topp and Byl, 1999; Barbe et al., 2003; Blake et al., 2005; Sommerich et al, 2007; Elliott et al., 2008). The movement dysfunction has been attributed to degradation of the somatotopic paw representation in the primary somatosensory cortex (S1) in owl monkeys (Byl et al., 1996, 1997; Topp and Byl, 1999). Using somatosensory evoked potential measurements, Byl et al. (2000, 2002) have also detected somatosensory disorganization consistent with somatotopic dedifferentiation in human subjects with severe and moderate focal hand dystonia. These observations have led to a learning based hypothesis as one origin of focal hand dystonia, and possibly other repetitive motion disorders. Based on this hypothesis, treatment interventions must be directed towards restoring normal cortical representations (Byl and Melnick, 1997; Byl and McKenzie, 2000; Byl et al., 2003; Candia et al., 2003; McKenzie et al, 2003).
Learning based treatment approaches are supported by studies in non-human primates. These studies provide evidence that coincident digital tactile inputs become co-represented in the S1 cortex, i.e. de-differentiating the finger representation. In theory, noncoincident repetitive inputs could restore normal segregation of the representation of contiguous skin territories, i.e. re-differentiation of the finger representation (Clark et al., 1988; Wang et al., 1995; Blake et al., 2005). In rat and primate studies, sensorimotor learning of a dexterity task produces drastic reorganization of movement representation and anatomical and functional properties of layer V cells in the primary motor cortex (M1) (Nudo et al., 1996; Kleim et al. 1998, 2002; Plautz et al., 2000; Remple et al., 2001).
In a rodent model, Barbe and Barr have reported the presence of a dose-dependent inflammatory response in peripheral forelimb musculoskeletal tissues in rats performing low to moderate repetitive reaching and grasping tasks (Barbe et al., 2003; Barbe et al, 2008; Elliott et al., 2008). In rats performing the low repetitive reaching task, the peripheral inflammatory response was mild, yet coincided with increased immunostaining for Substance P and its receptor, Neurokinin 1, in dorsal horns of the spinal cord, and with a decline in fine motor control (Elliott et al., 2008). The rats appeared unable to control the accurate placement of the forepaw on a target food pellet or to control the grasping of that pellet. As a result, repeated attempts were made by the animals to retrieve a food pellet, resulting in increased reversals of forelimb movement that resembled a raking motion. Rats performing moderate repetitive reaching tasks with negligible or high force grasping loads, also had increased neurochemicals in spinal cord dorsal horns that coincided with peripheral inflammatory responses (Elliott et al., 2009a,b). Although changes in fine motor control were not examined in these latter studies, declines in grip strength that negatively correlated with peripheral inflammatory responses were reported. In other studies using this model (Clark et al., 2003, 2004; Elliott et al 2009a), a peripheral nerve injury mechanism was identified in repetitive movement disorders. A nerve inflammatory response resulted in nerve tissue damage, fibrosis, and, subsequently, carpal tunnel syndrome (decreased nerve conduction velocity, forepaw sensation and grip strength).
Based on the above findings, the extent to which peripheral and central mechanisms coexist in the development of repetitive motion injury needs to be demonstrated as a first step in understanding their inter-relationship and the partitioning of their contribution to the causality of motor behavior decline. A model in which movement pattern changes, cortical reorganization in both S1 and M1 and peripheral inflammatory changes could be studied simultaneously would be enormously helpful to first clarify the co-existence of peripheral and central factors in repetitive motion disorders, In future experiments, the model could be adapted to study strategies for prevention as well as effective methods of intervention to remediate chronic inflammation and restore normal cortical topography.
We hypothesize that both central cortical reorganization and peripheral tissue injury mechanisms play a role in the development of functional disability caused by repetitive, hand-intensive task behaviors. Thus, in this current study, a rat model of repetitive motion injury was used to examine motor behavioral changes, signs of peripheral tissue inflammation and forelimb map organization (S1 and M1) associated with the performance of a reaching and grasping task that was moderately repetitive with negligible force (MRNF). This study has closed a significant gap in research by simultaneously monitoring motor performance, peripheral tissue inflammatory responses and electrophysiological cortical mapping as the first step in determining the interplay of multiple parallel mechanisms underlying repetitive motion injuries.
Materials and Methods
Animals
Twenty nine female, adult Sprague-Dawley rats (age 12–14 weeks at onset of experiment; Ace) were used. Experimental (EXP) rats (n = 12) were trained to perform a moderate repetitive, negligible force (MRNF) reaching and grasping task for 8 weeks. Age-matched normal control (CONT) rats (n = 14) were not trained to perform the task, but like EXP rats, underwent functional behavioral testing, electrophysiological cortical mapping and post-euthanasia tissue examination. Three additional trained-only control rats were included in the histological studies. Experiments were approved by the Temple University IACUC in compliance with NIH guidelines for the humane care and use of laboratory animals.
Repetitive task
EXP rats were placed in customized operant test chambers for rodents (Med. Associates, VT) with a portal and tube located in one wall. The tube was 2.5 cm in length so the shoulder had to be fully elevated and the elbow fully extended in order for the animal to reach pellets of food as described previously (Barbe et al., 2003; Barr et al., 2003; Clark et al., 2003). Purified formula food pellets (45 mg; Bioserve) were dispensed (Pellet dispenser, Med. Associates) every 15 seconds during the reach task.
The rats learned to reach for the food during an initial 10–12 day shaping period as previously described (Barbe et al., 2003 and Clark et al., 2003). During this period, the rats were food restricted for no longer than one week to no lower than 80% full body weight (as compared to CONT rats with free access to food). When they began to reach freely for the food, their diet was adjusted with rat chow to maintain them within ± 5% of full body weight. When they were able to perform the task consistently at no specified reach rate for 10 – 20 min/day, EXP rats were begun on the task regimen at the target rate of 4 reaches/min for 2 hrs/day, 3 days/wk for 8 wks. The force utilized to retrieve the 45 mg food pellet was estimated to be <5% maximum grasp force. The task was divided into 4, 0.5-hr sessions separated by 1.5 hrs in order to avoid satiation. Rats were allowed to use their preferred limb to reach. The side used to reach was recorded in each session. Thus, the animals were allowed to self-regulate their participation in task performance, which reduces possible pain and discomfort in the animals, and also makes this a voluntary task. There were 7 right dominant animals, 4 left dominant animals and one ambidextrous animal. For the ambidextrous animal, the forepaw tested for electrophysiological mapping was randomly selected. Two of the dominance-limbed rats switched limbs during the 12 weeks of task performance; in these rats, the cortices were mapped bilaterally. The trained control rats did not perform the MRNF past the initial training period.
Reach Behavior
Reach Performance
The number of reaches was logged by an observer using a hand-operated counter and reach distance criteria. A “reach” was defined as any time an EXP rat reached beyond and then withdrew the forepaw behind a line drawn 0.5 cm within the tube. Reach rate (RR) was defined as the average number of reaches performed per minute and was calculated on the last day of each week. Task duration was defined as the number of hours/day the rats participated in the task and was averaged over the 3 days within each week. Daily exposure (mean reaches per day) and total exposure performed over the weeks of task performance were also reported to clarify the cumulative workload. Daily exposure (mean reaches per day) was defined as the product of RR and task duration and was calculated on the last day of each week. Total exposure was the sum of the mean reaches per day across weeks of task performance. The percent of successful reaches (% Success) was calculated on the last day of each week by subtracting the number of food pellets dropped by the rats onto the floor pan of the operant training pen (inaccessible due to the wire mesh flooring) from the number of pellets dispensed during all four task sessions and dividing by the total number of reaches performed during those sessions.
Reach Movement Patterns
We also performed a quantitative movement pattern analysis of reach movement reversals using a video camera to record the reach limb from a lateral view, as described and diagramed in more detail in Elliott et al., 2008. Briefly, EXP animals were videotaped on the last day of weeks 1 and 8 during 15 minutes of task performance. The 15-minute video segment was divided into 5 equal (3 minute) sections. Five representative reaches were selected from these segments from weeks 1 and 8. A representative reach (successful) was defined as a reach sequence beginning with the reach paw in a fixed position and the snout in the tube opening, followed by the paw entering the portal and retrieving a food pellet, and ending with the consumption of the food pellet. The time of this entire sequence was defined as the reach phase time (RPT). The pellet retrieval time, termed the grasp phase time (GPT), was defined as the time between when the reach forepaw first enters the tube opening until the time when the reach forepaw exits the tube opening with the food pellet. The number of extra elbow movement reversals (MR; fore-aft movements of the forelimb) made was used as an indicator of diminished fine motor coordination, as described in detail in Elliott et al, 2008. Gross movement patterns were also examined for deviations from the normal movements comprising reaching in rats (Wishaw et al., 1990).
Two distinct alternative movement patterns were classified. Scooping referred to a pattern in which the semi-open forepaw was placed over the food pellet and the pellet was dragged along the bottom or side surface of the tube and scooped into the mouth. Raking was an inefficient extreme of scooping in which repeated unsuccessful attempts to contact the food pellet with the semi-open forepaw resulted in repeated back and forth movements that resembled a raking motion. These behaviors were noted as present (>1/min) or absent on the last day of each week. They were also expressed as the percentage of animals in which the behaviors were present.
Functional strength and agility
Forepaw grip strength
Grip strength of the preferred reach limb was tested in EXP and CONT rats weekly as previously described (Clark et al., 2003). The test was repeated 3 to 5 times on the reach forelimb, and the average maximum grip strength was computed.
Forelimb Agility
The forehead sticker removal (FHSR) test was used to determine functional agility. This fine motor grooming skill was determined at the end of the final week of task performance in EXP rats and just prior to euthanasia in CONT rats. Rats were placed in a plastic, standard rodent home cage. A 2 cm diameter removable adhesive label (Avery) was placed on the forehead centered between the eyes and ears. Rats were observed for 3 minutes for sticker removal performance and graded on a 6-point scale (0 = no attempt at sticker removal to 5 = successful removal of sticker with one or both forepaws; Schrimsher and Reier, 1992). The test was repeated 5 times and an average score was computed.
Electrophysiological Mapping Procedures
Fourteen CONT and 12 EXP rats underwent electrophysiological mapping sessions 5–12 days after the end of the task training. The forepaw representation in the primary somatosensory cortex (S1) contralateral to the trained arm was mapped in 7 EXP rats; the S1 maps were mapped bilaterally in 2 EXP rats. The S1 forepaw map was derived in the left hemisphere in 8 control rats. The movement representation of the trained arm was mapped in the contralateral primary motor cortex (M1) in 7 EXP rats, two of which were also used for somatosensory cortical mapping. The M1 forelimb representation was mapped in 6 control rats. The investigators mapping the forepaw representation in either the S1 or M1 cortex were unaware of the experimental conditions of the animal throughout the electrophysiological session. The reproducibility of receptive fields (RF) delineation, movement assignment and map elaboration was frequently checked between experimenters.
Somatosensory maps
Each animal was anesthetized with sodium pentobarbital (50 mg/ kg, i.p.) and supplemental doses (5 mg/kg, i.p.) were injected as needed to maintain a deep level of anesthesia. Repeated injections of lactated Ringers solution allowed the animal to be kept in stable physiological conditions during the mapping session. The rectal temperature was maintained near 37.5°C with a thermostatically controlled heating pad. A craniotomy was made over the S1 cortex at a site corresponding to the forelimb representation (between −1 and +3 mm from bregma in the rostrocaudal axis and between 1 and 4 mm in the mediolateral direction). After resection of dura mater, the surface of the cortex was covered with a thin layer of warm silicone fluid to prevent drying and edema. An enlarged image of the exposed brain surface was used to guide and record the location of electrode penetrations in MAP software (Peterson and Merzenich, 1995). Multiunit recordings were made with tungsten microelectrodes (1MΩ at 1 kHz, WPI, FL) introduced perpendicular to the cortical surface. The interelectrode penetration distance ranged from 50 to 100 µm and was roughly similar for all rats. The recording artifact generated by the microelectrode contact with the cortical surface was set to zero. The electrode was advanced into the cortex using a hydraulic microdrive (David Kopf Instruments, Tujunga, CA); receptive fields (RFs) were determined in the upper layer IV (650–800 µm) of the S1 cortex.
The sensory mapping procedure used has been previously described (Coq and Xerri, 1998, 1999). Briefly, the multiunit signal was preamplified, filtered (bandpass: 0.5–5 kHz), displayed on an oscilloscope, and delivered to an audio monitor. At each recording site, S1 cortical responses produced by natural stimulation were detected by large bursts of activity. Low-threshold cutaneous RFs were defined using hand-held probes or brushes to produce light skin indentations or gentle hair movements. High-threshold cortical sites were classified as responses elicited by taps on skin and stroking hairs. Responses to manipulations of muscles and joints were presumed to be related to deep receptors. Responses evoked by nail movements were examined while the tips of the digits were firmly stabilized to minimize joint movement and skin deformation. Cortical responsiveness to somatic stimuli was classified along a 3-level scale (weak, good and excellent responses) on the basis of the magnitude of the signal-to-noise ratio, as previously described (Coq and Xerri, 1998, 1999). Unresponsive cortical sites exhibited spontaneous discharges only.
The ridges of the glabrous skin of the digits and palm were used as reliable landmarks to delineate RFs. The RF areas were transferred to the forepaw digital image and then measured offline using MAP software. Canvas software (Deneba) was used to elaborate maps of the forelimb representation by drawing boundaries encompassing cortical sites whose RFs were restricted to a common forelimb subdivision (e.g. digit, palmar pad, base of the forepaw, and forearm). Borders were drawn midway between adjacent recording sites when RFs were located on distinct and separate skin subdivisions. A boundary line crossed cortical sites when a single RF included different but adjoining skin subdivisions of the forelimb. Map borders were placed midway between responsive and unresponsive sites.
Motor maps
Standard intracortical microstimulation (ICMS) techniques were used to derive detailed maps of the M1 movement representation of the contralateral arm involved in the reaching task (Kleim et al., 1998; Strata et al., 2004). Prior to surgery, animals were anesthetized with ketamine hydrochloride (70 mg/kg i. p.) and xylazine (5 mg/kg i. p.). Supplementary doses of ketamine (20 mg/kg i. p.) and acepromazine (0.02 mg/kg i. p.) were delivered as needed to maintain a deep and constant level of anesthesia. A craniotomy was made over the M1 cortex at a site corresponding to the forelimb motor representation (between −6 and +1 mm from bregma in the rostrocaudal axis and between 1 and 6 mm in the mediolateral direction). After resection of dura mater, the surface of the cortex was covered with a thin layer of warm silicone fluid to prevent drying and edema. An enlarged image of the exposed brain surface was used to guide and record the location of electrode penetrations in MAP software. Microelectrode (100 kΩ, FHC, ME) penetrations were at a depth of about 1,700 µm spaced 100–200 µm apart, depending on the surface blood vessel patterns. Microstimulations consisted of 40 ms trains of 200-µs biphasic cathodal pulses delivered at 333 Hz from an electrically isolated, constant current stimulator (A-M Systems, WA). Pulse trains were delivered at about 1 Hz. At each site, the stimulation was initiated at the lowest intensity (5 µA) and was gradually increased until a forelimb movement was evoked. Joint movements of the digits, wrist, elbow and shoulder and other body parts were visually defined. Sample responses were recorded with a digital videocamera for post hoc movement confirmation. If no movement was elicited at 60 µA, the site was defined as “non-responsive”. We used Canvas to reconstruct maps of the forelimb representation by drawing boundaries encompassing cortical sites whose movements were elicited from the same joint (shoulder, elbow, wrist, fingers) or combinations of joints (e.g. elbow-wrist, arm-fingers).
Cytokine analyses of muscles and tendons
ELISA detection of TNF-α and IL-1β. Four EXP rats and 4 control (CONT) rats were euthanized with sodium pentobarbital (Nembutal; 120 mg/kg body weight). This method is consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. Flexor digitorum muscles and tendons were collected separately and flash frozen at −80°C until homogenization. Later, they were homogenized and lysates analyzed for IL-1β and TNF̣-α using commercially available ELISA kits (BioSource International) and methods described previously (Barbe et al., 2008). Each sample was run in duplicate and data (pg cytokine protein) normalized to µg total protein.
Immunohistochemical analysis of TNF-α and IL-1β. Eight EXP and 4 normal control rats that had undergone cortical mapping, as well as 3 trained control rats, were studied immunohistochemically for the presence of TNF-α and IL-1β immunopositive cells in flexor forelimb (flexor digitorum) muscles, tendon and nerve. These rats were euthanized by lethal overdose (Nembutal, 120 mg/kg body weight) and perfused transcardially with 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4). Flexor forearm tissues were cryosectioned and immunostained using methods described previously (Barbe et al., 2003; Al-Shatti et al., 2005). Forearm muscle changes were quantified using methods described previously (Barbe et al., 2003; Al-Shatti et al., 2005). No differences were found in the number of immunostained TNF-α or IL-1β cells in muscle or tendon in normal versus trained control rats (p>0.05), therefore these counts were combined for statistical and graphing purposes and termed CONT.
Statistical Analysis
All dependent variables were described (mean, standard error of the mean). Repeated measures ANOVAs were used to determine differences in percent successful reaches, reach rate, and task duration across weeks in EXP rats, followed by Bonferroni post hoc tests. Paired samples t-tests were used to determine differences in number of movement reversals, RPT, GPT and grip strength between week 1 and week 8 in EXP rats, and grip strength in CONT rats (a measurement used for correlations). A Mann-Whitney U test was used to determine the difference in forehead sticker removal test scores between CONT and week 8 EXP rats.
For the cortical map data, Canvas software (Deneba) was used to calculate the areal extent of each region of the S1 and M1 cortical maps. Cortical zones were described by their absolute areas (mm2) for each rat. For the S1 maps, the absolute cortical zones devoted to the representation of either glabrous or hairy skin surfaces were normalized relative to the ventral and dorsal forepaw skin surfaces, respectively. Average values were computed for each group of rats. The absolute size of glabrous and hairy RFs, measured in mm2, was normalized relative to the ventral and dorsal forepaw skin surfaces, respectively, and expressed in percentages. Since several RFs encompassed glabrous and dorsal forepaw skin, the RF area was also normalized relative to the sum of the ventral and dorsal forepaw skin surfaces. The relative RF areas measured in each rat were averaged and the mean size of the RFs computed in each group. The RF average size per rat was calculated by including or not the large RFs located on the dorsal surface of the forepaw. S1 and M1 map data were analyzed using Mann-Whitney U-tests and chi square tests.
Two-way ANOVAS were used to determine the differences in TNF-α and IL-1β levels in flexor muscles and tendons, and the number of TNF-α and IL-β immunopositive cells in flexor muscles, with the factors group (CONT and week 8) and limb (preferred reach and contralateral nonpreferred) for each cytokine, followed by a Bonferroni post hoc test. All correlations were performed using Spearman’s rho rank correlations with R (The R Foundation for Statistical Computing) to determine the relationship between sensorimotor cortical measures, behavioral variables and inflammation. The ratio between week 1 and week 8 EXP behavioral outcomes and between CONT and week 8 EXP inflammation outcomes were used in these correlation tests when comparing cortical findings to behavioral or inflammation data.
Results
Task performance, forelimb strength and agility
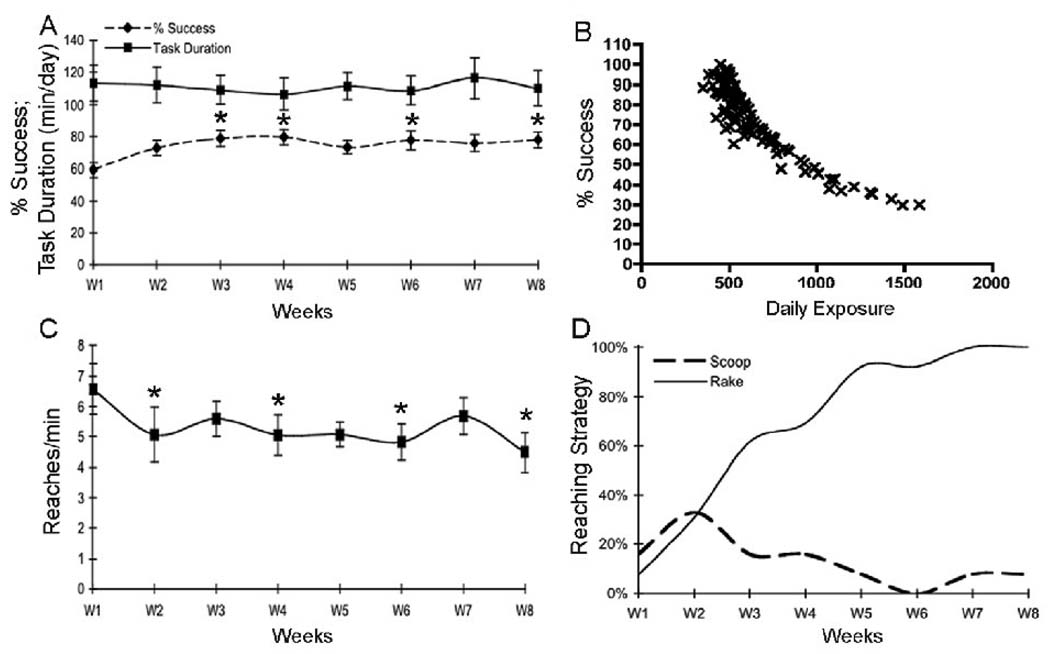
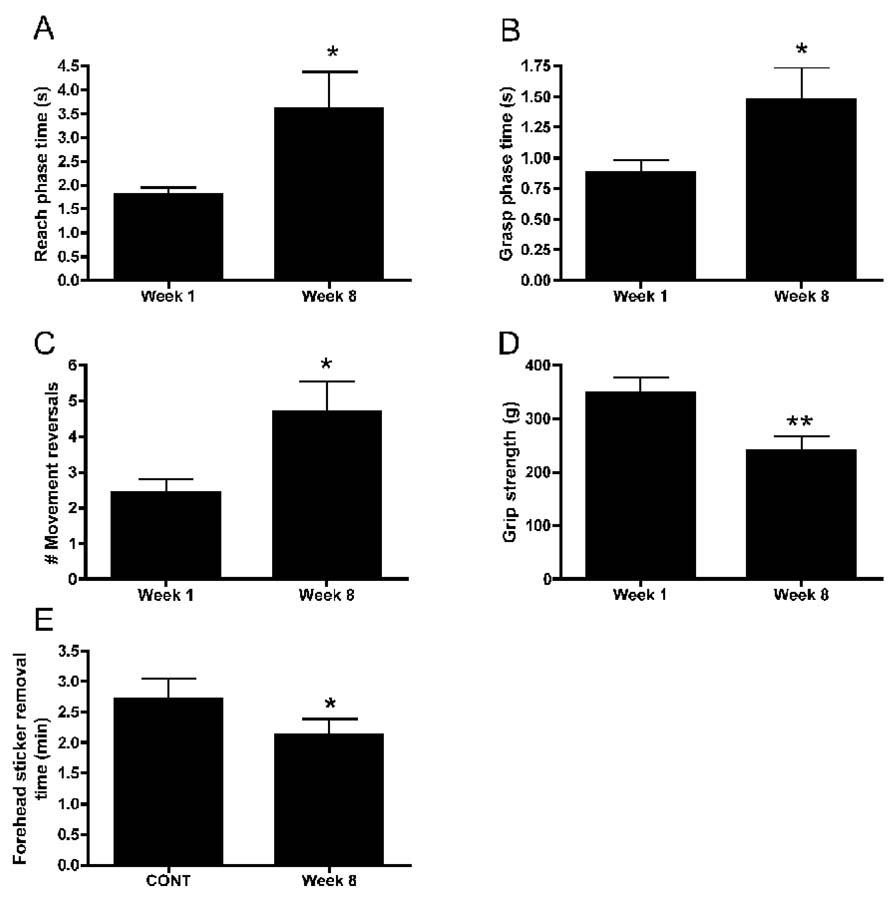
The mean daily exposure (mean reaches/day) was 627±18.70 (mean±SEM; range 202 to 1584), while the cumulative total exposure in EXP rats was 7123±1258 reaches (range of 3159 to 19646). The percentage of successful reaches increased in weeks 3, 4, 6 and 8 compared to week 1 (p<0.05 each; Fig. 1A dotted line). In contrast, task duration (Fig. 1A solid line) and daily exposure (product of reach rate and task duration; data not shown) were not significantly different across weeks of task performance. However, there was a significant negative correlation between the percent of successful reaches per day and mean daily exposure (r=−0.87; p<0.0001; Fig 1B), indicating the rats with the greatest daily workload had the lowest success rate. There were anticipated and significant decreases in reach rate in weeks 2, 4, 6 and 8 compared to week 1 (p<0.05 each; Fig. 1C). The proportion of rats using raking as a reach movement strategy was clearly larger in week 8 (100%) than in week 1 (<10%; Fig. 1D). The proportion of rats using scooping as a reaching strategy nearly doubled between week 1 and 2, but then declined as raking replaced it as the main reaching strategy (Fig 1D). There were increases in the reach phase time (p=0.02), grasp phase time (p=0.04) and number of movement reversals (p=0.03) in week 8 compared to week 1 (Fig. 2A–C), each indicative of a decline in fine motor control and/or agility. As reported previously, the extra movement reversals were corrections for missed food pellets during a reach (Elliott et al., 2008). This increase in reach phase time correlated negatively with the percentage of successful reaches (r=−0.69; p=0.006), also suggestive of a decline in fine motor ability. In terms of functional motor outcomes, there were significant decreases in grip strength in week 8 compared to week 1 (p=0.008; Fig. 2D), and FHSR scores in week 8 compared to normal controls (p=0.05; Fig 2E). Since the FHSR rest is a grooming test indicative of forepaw abilities, its decline is indicative of a decrease in forepaw agility. Thus, the MRNF task led to several motor behavioral deficits, decreased agility, and movement strategy changes by week 8 of task performance.
Fig 1.
Behavioral variables showing changes across 8 weeks of moderate repetition, negligible force (MRNF) task performance. (A) Percentage of successful reaches in which a food pellet was retrieved increased in weeks 3, 4, 6 and 8 compared to week 1. Task duration did not change significantly over time. (B) Spearman’s correlation showing strong negative correlation between mean daily exposure (mean reaches/day) and % Success (r=−0.87). (C) Reach rate declined in experimental animals in weeks 2, 4, 6 and 8 compared to week 1. (D) The proportion of experimental rats using either scooping or raking as a food retrieval strategy. All rats used raking as their main strategy of food retrieval by week 8. Plots A and C show means ± SEM. *:p<0.01 compared to week 1.
Figure 2.
Behavioral variables showing changes in week 8 compared to week 1 with moderate repetition, negligible force (MRNF) task performance. (A) Reach phase time (total time in seconds (s) of the entire reach sequence from forepaw elevation to food pellet consumption), (B) grasp phase time (i.e., time in seconds from the entrance of the forepaw into the portal until withdrawal of the forepaw from the portal after pellet retrieval), and (C) number of reach movement reversals increased in experimental animals in week 8 compared to week 1. (D) Forepaw grip strength in grams (g) and (E) forehead sticker removal time in minutes (min) decreased in week 8 compared to week 1 or normal controls (CONT). Plots show means ± SEM. *: p<0.01 compared to week 1 or normal controls, as indicated in graph.
S1 maps
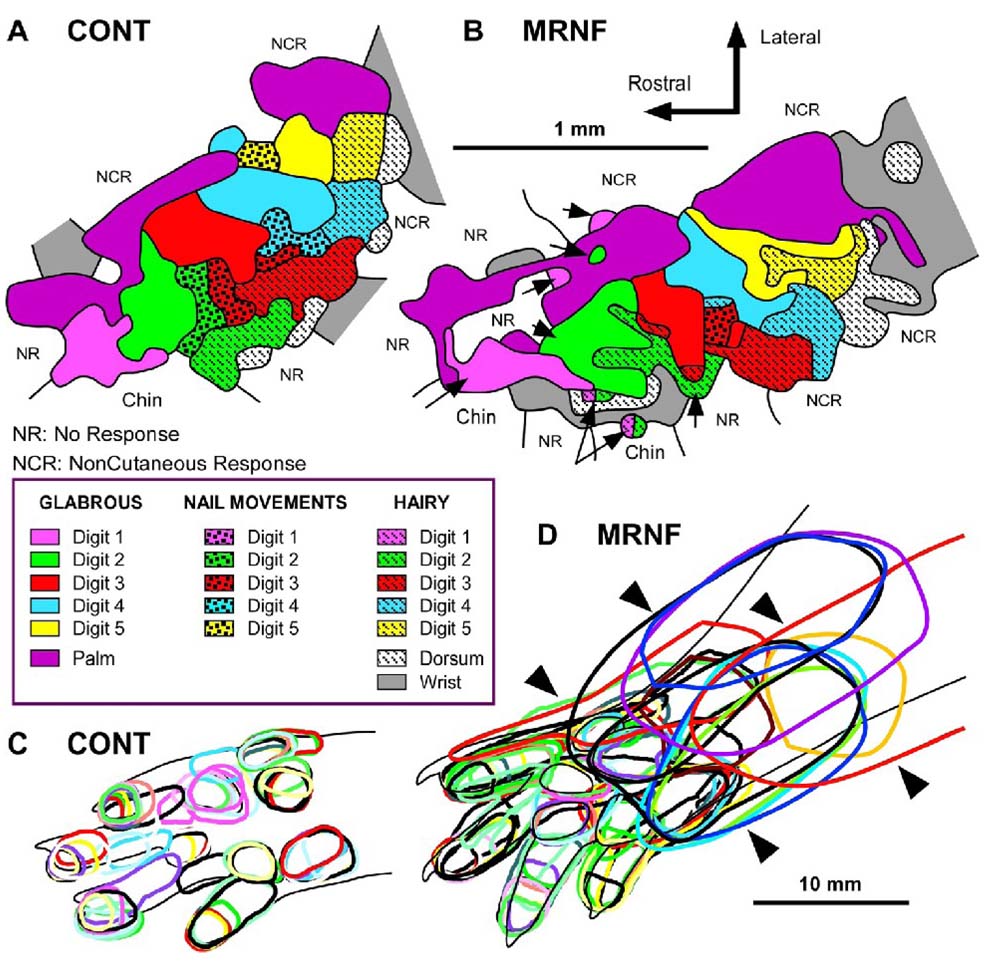
Nine electrophysiological maps of the forepaw skin representation were derived in the S1 cortex from a total of 2320 electrode recordings, averaging 137±33 cortical penetrations per map. As shown in previous studies (Coq and Xerri, 1998, 1999), the S1 forepaw representation in rats displays invariant organizational features despite inter-individual differences. Briefly, the cortical forepaw map is somatotopically organized in the rostrocaudal direction, from the thenar eminence and digit 1 to the hypothenar eminence and digit 5, and in the mediolateral direction, from the palmar pads to the glabrous and hairy skin surfaces of digits (Fig. 3A). Cortical zones responding to nail movements are inserted between those serving the glabrous and hairy skin. The external boundaries of the forepaw cutaneous map are consistently delimited by noncutaneous responses, chin representation in the rostrolateral portion, and wrist-forearm representation in the caudal portion of the forepaw map. The moderate repetition, negligible force (MRNF) reaching task induced a topographical disorganization of the S1 forepaw maps, characterized by a disruption of the representation of contiguous skin surfaces and many RFs encompassing the forepaw and the forearm, while the overall somatotopy was preserved (Fig. 3B).
Figure 3.
Primary somatosensory (S1) cortical maps in control (CONT) and experimental (EXP) rats performing a moderate repetition, negligible force (MRNF) task. (A) Representative map in a normal control rat. (B) Representative map in an 8 week MRNF rat showing disruptions in the continuity of the S1 representation of contiguous forepaw skin surfaces and patchy representations of single forepaw subdivisions (e.g. digits 1 or 2; examples indicated by arrows) into distinct cortical zones. (C) Typical glabrous receptive fields in a normal control rat showing single receptive fields (RFs) encompassing mainly one or two forepaw subdivisions. (D) Glabrous RFs in a MRNF rat showing many large RFs encompassing several forepaw subdivisions and even larger RFs located on both plantar pads or digits and wrist/forearm (examples indicated by arrowheads). Note the greater overlap of glabrous RFs in EXP rats (D) than in CONT (C).
The total area of the S1 forepaw representation did not differ between CONT and EXP (U=30.0; p: n. s.), nor did the glabrous or hairy representations (U=26.0; p: n. s.) of the forepaw (Table 1). We found similar results when the cortical area was normalized relative to the forepaw skin area (data not shown). Although the size of the S1 map did not change, it was positively correlated with the ratio of grip strength in week 1 versus 8 (r=0.83, p=0.015). Also, the proportion of cortical sites responding to both forepaw cutaneous stimulation and nail movements was greater (χ2=18.53; p<0.0001) in EXP (290/712; 40.7%) than in CONT (179/701; 25.5%). This result shows a change in neuronal selectivity between cutaneous and nail movement inputs and emphasizes the repetitive task-induced alterations of the S1 neuronal properties. In addition, the “cortical islets” devoted to represent nail movements, located between the representations of glabrous and hairy forepaw skin surfaces found in CONT tended to disappear in EXP rats. In fact, nail movement responses were found to be mixed with cutaneous responses within the same S1 cortical site in the EXP group (Fig. 3B), also reflecting a disruption of the S1 map.
Table 1.
Cortical area occupied by forepaw skin regions and relative receptive field (RF) size in controls (CONT) and experimental rats (EXP, moderate repetition negligible force). Mean±SD is shown.
| CONT | EXP | |
|---|---|---|
| Total area (mm2) | 1.95±0.29 | 1.89±0.39 |
| Glabrous skin (mm2) | 1.34±0.22 | 1.42±0.29 |
| Hairy skin (mm2) | 0.54±0.13 | 0.47±0.15 |
| All RFs (%) | 4.5±1.2 | 5.28±1.2 |
| RFs w/o forepaw dorsum (%) | 3.6±0.8 | 4.7±0.9a |
| RFs on glabrous skin (%) | 5.7±1.7 | 8.3±1.7b |
| RFs on hairy skin (%) | 14.1±4.5 | 14.4±4.4 |
Significantly different from controls, p<0.02.
Significantly different from controls, p<0.01.
The MRNF task altered the proportions of RFs assigned to “single” or “multiple” categories (χ2=24.92; p<0.0001). EXP rats exhibited a larger proportion (157/712; 22.1%; Fig. 3D) of single RFs encompassing several forepaw subdivisions (i.e. digits and/or palmar pads) than in CONT (91/701; 13.0%; Fig. 3C), while both groups had about the same numbers of single RFs located on one subdivision (i.e. one digit or one pad). The proportion of multiple RFs (i.e. cortical sites receiving inputs from disjunctive skin surfaces) was lower in EXP (58/712; 8.1%) than in CONT (90/701; 12.8%). Instead, significantly more cortical sites exhibiting RFs located on both glabrous and hairy surfaces were found in EXP (109/712; 15.3%) than in CONT (26/701; 3.7%; χ2=45.58; p<0.0001). These changes also reflect the degradation of S1 neuronal properties after the MRNF task.
Since a large number of RFs encompassed both glabrous and hairy surfaces of the forepaw, the RF area was normalized relative to the sum of the ventral and dorsal forepaw skin surfaces. The mean size of all RFs did not differ between groups, but when the large RFs for dorsal forepaw were discarded, the mean size of the remaining RFs was significantly larger in EXP than in CONT (U=11.0; p<0.02; Table 1). In addition, the RFs located on the glabrous forepaw were 1.5 times larger in EXP than in CONT (U=9.0; p<0.01), whereas the size of hairy RFs did not differ between groups (U=34; p: n.s.; Table 1). The larger the glabrous RFs on the forepaw, the lower the mean percent of successful reaches (see Table 3). Figure 3B illustrates the degradation of the topographic organization of the S1 forepaw maps after the repetitive task. There was an enlargement of the overall size of RFs, a greater overlap of glabrous RFs, and more RFs located on both glabrous and hairy sides of the forepaw in EXP than in CONT (Fig. 3A versus B). The forepaw representation appeared to be patchy and the S1 map topography was disrupted in EXP relative to controls (Fig. 3A versus B). For example, disruptions in the continuity of the S1 representation of contiguous skin surfaces of the forepaw and discontinuous representations of several single forepaw subdivisions into distinct cortical zones within the S1 forepaw map (see the patchy representation of digits 1, 2 and 3 in Fig. 3B), features not seen in CONT S1 maps.
Table 3.
Statistically significant Spearman’s rank correlation coefficients (r) for primary somatosensory (S1) and motor (M1) cortical maps, behavior and inflammation.
| RPT e | TNFα | |||
|---|---|---|---|---|
| S1 or M1 map results | % Success | % Raking | Ratio f | Ratio f |
| Muscleg | ||||
| S1 glabrous RFs size (%) | −0.72a | n.s. | n.s. | n.s. |
| S1 RFs on forearm size (mm2) | −0.89 b | 0.8 b | n.s. | n.s. |
| S1 RFs on forearm size (%) | −0.84a | 0.9 b | 0.9 b | n.s. |
| M1 multi-jointc area (%) | n.s. | 0.97 b | n.s. | n.s. |
| M1 elbow area (mm2) | n.s. | n.s. | n.s. | 1.0 a |
| M1 wrist threshold (µA) | n.s. | n.s. | n.s. | −0.89 b |
| M1 elbow-Wrist threshold (µA) | n.s. | n.s. | n.s. | −0.86 a |
| M1 arm-digit dthreshold (µA) | n.s. | n.s. | n.s. | −0.89 b |
Statistically significant, p<0.05.
Statistically significant, p<0.01.
Multi-joint = elbow-wrist + arm-digits
arm = shoulder-elbow-wrist
RPT = reach phase time
Ratio = ratio of week 1 results compared to week 8 results
Muscle = refers to flexor digitorum
Another conspicuous feature in the S1 forepaw maps of EXP rats was the higher proportion (χ2=19.10; p<0.0001) of RFs located on both palmar pads, digits or dorsal forepaw and wrist or forearm in EXP (81/134; 60.5%) than in CONT (30/99; 31.3%; Fig 3). As shown in Table 3, these large RFs located in the forepaw and forearm correlated negatively with the percentage of successful reaches, positively correlated with percentage of rats using the inefficient food retrieval pattern of raking, and positively correlated with the reach phase time ratio of week 1 to week 8.
In an attempt to gain insight into the possible behavioral task-induced changes in the response sensitivity of S1 neurons, the responses to just-visible skin indentation were classified along a 3-level ordinal scale (weak, good and excellent responses) on the basis of the magnitude of the signal-to-noise ratio, as in previous studies (Coq and Xerri, 1998, 1999). The proportions of weak (87/594; 14.7%) and good (249/594; 41.9%) responses were slightly decreased in EXP compared to the weak (117/701; 16.7%) and good (392/701; 55.9%) responses in CONT. In contrast, the proportion of excellent responses was greater in EXP (258/594; 43.4%) than in CONT (192/701; χ2=37.14; p<0.0001). Similar results were found when good responses were discarded from the analysis since it represented the dominant category (χ2=13.04; p<0.0003). Thus, the MRNF task increased the cortical responsiveness to light tactile stimulation, another change in the S1 neuronal properties related to the MRNF task.
M1 maps
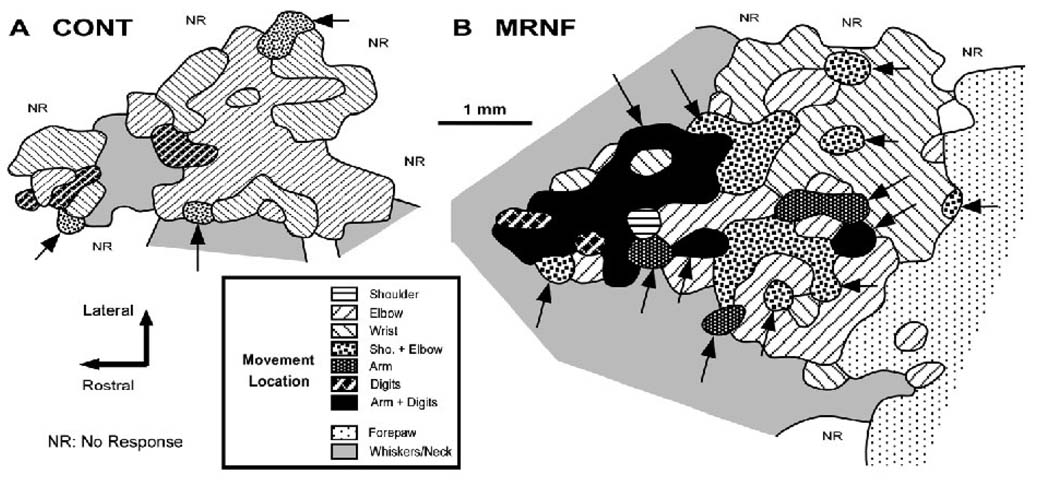
Cortical maps of the movement representation of the forelimb were derived in the primary motor cortex (M1) of 7 EXP and 6 CONT rats by intracortical microstimulation techniques. Maps were generated from a total of 2074 electrode penetrations, averaging 160±32 penetrations per map. In all CONT rats, the rostral forelimb area (RFA) was clearly separated from the caudal forelimb area (CFA) by the representation of the whiskers, jaw or neck, as found in previous motor mapping studies (e.g. Neafsey et al., 1986; Nudo et al., 1990; Kleim et al., 1998). The M1 forelimb area was delimited by either no responses or by whisker, jaw or neck movements in the rostral, medial and lateral borders and by hind limb movements in the caudal border. In CONT, both RFA and CFA contained representations of the shoulder, elbow, wrist and digits (not in all cases) that were not clearly topographically distributed (Fig. 4A), as described in previous studies (Nudo et al., 1990; Kleim et al., 1998). In CONT, the shoulder representation occupied an average of 3% of the total M1 forelimb area, 49% was the elbow, 31% the wrist and 2% the digits. In CONT, 23.3% (137/587) of M1 cortical sites evoked multi-joints responses, such as elbow-wrist (9% of the total map area), arm (shoulder-elbow-wrist, 5%), and arm associated with digit flexion (1%; Table 2).
Figure 4.
Primary motor (M1) cortical maps in control (CONT) and experimental (EXP) rats performing a moderate repetition, negligible force (MRNF) task. (A) Representative map in a normal control rat. (B) Typical map in an 8 week MRNF rat showing a drastic enlargement of the M1 forelimb cortical map and the task-induced increase in the cortical area devoted to digit, arm-digit (arm = shoulder-digit-wrist) and multi-joint movements (multi-joint responses are highlighted by arrows).
Table 2.
Cortical area (mm2) occupied by forelimb movements in experimental (EXP; moderate repetition, negligible force) and control (CONT) rats, and mean thresholds (µA) to evoke movements. Mean±SD is shown.
| Movement | CONT | EXP |
|---|---|---|
| Total map area | 6.74±0.49 | 10.62±2.19b |
| Shoulder | 0.18±0.16 | 0.55±0.47 |
| Elbow | 3.31±1.52 | 4.31±1.39 |
| Wrist | 2.06±0.82 | 2.46±1.23 |
| Digits only | 0.11±0.26 | 0.15±0.12 |
| Elbow–Wrist | 0.60±0.60 | 1.79±0.75a |
| Arm c | 0.87±0.08 | 0.37±0.41 |
| Arm–Digits c | 0.06±0.02 | 0.99±0.81b |
| Multi-joint d | 0.95±1.13 | 3.15±1.53a |
| Overall map threshold | 30.2±10.2 | 28.0±6.0 |
| Shoulder threshold | 26.5±15.4 | 29.4±16.8 |
| Elbow threshold | 30.4±10.4 | 29.5±8.7 |
| Wrist threshold | 32.7±12.2 | 26.7±7.4 |
| Digit threshold | 37.5± 9.1 | 18.0±10.1a |
| Elbow-Wrist threshold | 30.7±12.4 | 26.1±9.4 |
| Arm threshold c | 28.7±17.0 | 29.8±15.9 |
Significantly different from controls, p<0.05.
Significantly different from controls, p<0.01.
Arm = shoulder-elbow-wrist multi-joint movement representation
Multi-joint = elbow-wrist + arm-digits
The MRNF reaching task drastically increased the total area of the M1 forepaw movement representation (1.6 times larger) compared to CONT (U=0; p=0.006; Fig 4B; Table 2). The areas occupied by the shoulder (6% of the total map area), elbow (41%), wrist (23%), digits (1%) and arm (3%) were not significantly affected by the MRNF task (Table 2). Instead, the movement representation area (mm2) of the elbow-wrist multi-joint responses tripled (U=2.0; p=0.02; 17% of the total map area) and the arm-digits multi-joint areal extent was 17 times larger than in CONT (U=1.0; p=0.01; 9% of the total map area; Table 2). Overall, the proportions of cortical sites that elicit multi-joint movements increased in EXP rats (167/510; 32.8%) relative to CONT (137/587; 23.3%; χ2=12.05; p=0.0005). As shown in Table 2, the cortical area devoted to multi-joint movements increased in EXP rats (29% of the total map area) relative to CONT (U=3.0; p=0.03; 14% of the total area). Interestingly, the cortical increase in the multi-joint movement representation for elbow-wrist, arm and arm-digits correlated strongly with the increased prevalence of the raking strategy, as well as the elbow movement representation (Table 3).
The overall threshold (i.e. minimal current to evoke a movement) of neuronal M1 responses did not differ between groups (U=15.0; p: n.s.) nor did the threshold for most forelimb joints, except for digits (digits only and digit-arm), which was 2 times lower in EXP than in CONT (U=1.0; p=0.03; Table 2). As the arm-digit representation was found only in one control rat, the arm-digit and digit only threshold values were pooled together as digit. Out of the motor forepaw map, the average thresholds required to evoke whisker and hind paw movements did not differ between EXP (27.6±6.4 µA) and CONT (27.5±8.0 µA) rats (U=21.0; p: n.s.), so that the MRNF task specifically altered the M1 digit representation. In addition, relatively similar proportions of cortical sites with weak, clear and strong movement responsiveness for forelimb movements (χ2=0.36; p: n.s.), hind limb, and whiskers (χ2=0.85; p: n.s.) were found in both groups. Thus, the MRNF task drastically increased the size of the M1 forepaw maps, especially the movement representation of the digits, digit-arm and elbow-wrist specifically involved in the behavioral task, and decreased the amount of current required to evoke movements of the digits.
Forelimb flexor muscle and tendon inflammation
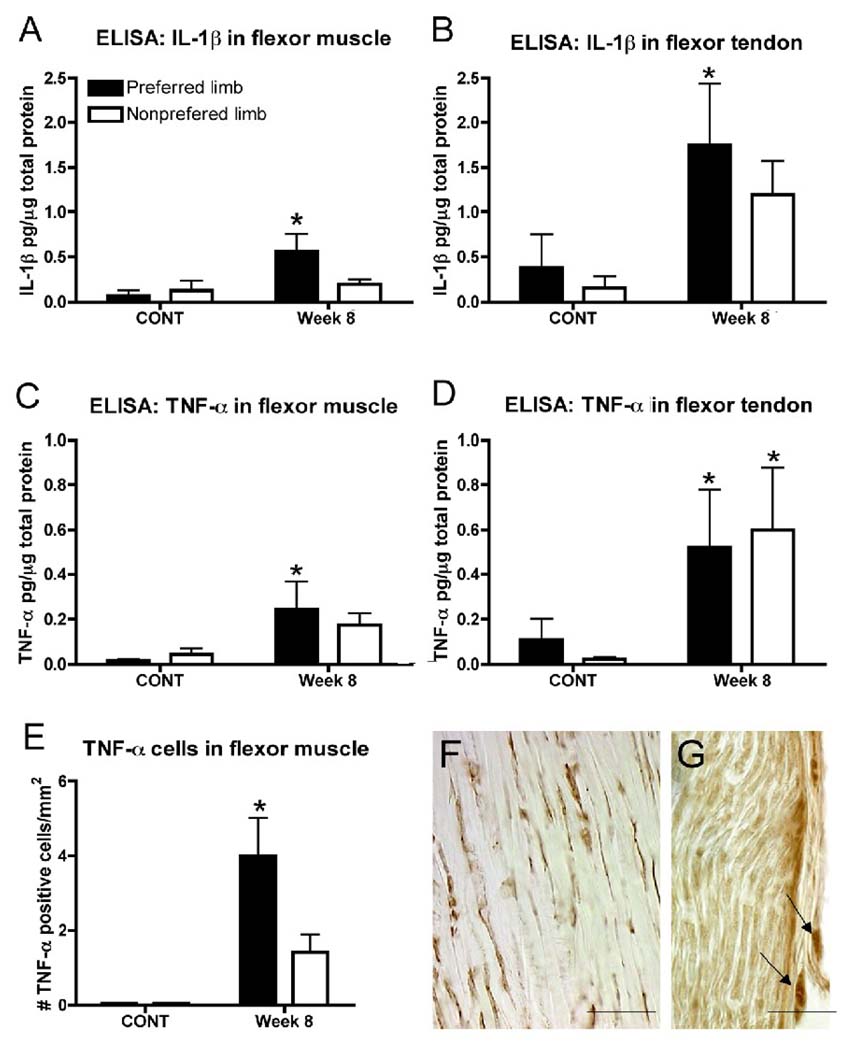
TNF-α and IL-1β increased in forelimb flexor muscles and tendons with performance of the MRNF task (Fig. 5). There were significant increases of IL-1β in EXP week 8 preferred reach limb flexor muscle and tendon (p<0.05 for each) compared to CONT rats (Fig. 5A and B). There were also significant increases of TNF-α in EXP week 8 preferred reach limb flexor muscle and tendon (p<0.05 for each) compared to CONT rats (Fig. 5C and D) as well as in the contralateral, nonpreferred limb tendon (Fig. 5D). When examining flexor forelimb muscles immunohistochemically, the number of cells expressing TNF-α in flexor muscle of the preferred reach limb was significantly increased in EXP compared to CONT rats (p=0.01; Fig. 5E). TNF-α immunopositive cells were also present in forelimb flexor tendon and median nerve at the level of the wrist (Fig. 5F and G), cells not observed in CONT rat tissues (data not shown).
Figure 5.
Inflammatory mediators detected in experimental rats after 8 weeks of moderate repetition, negligible force (MRNF) task performance as compared to controls (CONT). (A, B) ELISA levels of IL-1β protein in forelimb flexor digitorum muscle and tendon in preferred reach limbs of week 8 MRNF rats increased compared to normal controls. (C, D) ELISA levels of TNF-α protein in flexor digitorum muscle and tendon in preferred reach limbs of week 8 MRNF rat increased compared to normal controls, and in the nonpreferred limb tendon, compared to normal controls. (E) The number of TNF-α immunopositive cells in flexor digitorum muscles of preferred reach limbs of week 8 MRNF animals increased compared to normal and trained control rats (mean levels of both combined). Plots show means + SEM; *:p<0.05 compared to controls. (F, G) TNF-α immunopositive cells (brown DAB reaction product) in the flexor digitorum forelimb tendons (F), and median nerve (G) of a representative preferred reach limb of a week 8 experimental animal. Arrows in G indicate fibroblasts in extraneural connective tissue. Scale bar = 50 µm.
Using Spearman’s correlations, we observed a negative correlation between the ratio of grip strength in weeks 1 versus 8 in EXP rats and TNF-α levels in tendon (r=−0.85; p=0.006) and muscles (r=−0.72; p=0.017), and IL-1β levels in muscles (r=−0.71; p=0.02). Thus, the MRNF task led to increased inflammatory responses in muscle, tendon and nerve, changes that affected grip strength, which declined over time (see Fig. 2D). Table 3 also shows that the increased inflammatory response in muscles of MRNF rats had a strong negative correlation with the amount of current required to evoke movements of the wrist, and elbow-wrist, and arm-digit multi-joints. The higher the inflammation in flexor muscles specifically involved in the task, the lower the threshold required to elicit arm-digit movements, which decreased in EXP rats relative to controls.
DISCUSSION
The results from this study contribute to our understanding of the complexity of repetitive motion disorders by showing, for the first time, the coexistence of both peripheral inflammatory and central neuroplastic mechanisms associated with motor behavior declines in a unique model of repetitive motion injury. In these experiments, rats performed a voluntary moderate repetitive, negligible force (MRNF) reaching task that has been demonstrated previously to cause localized injury and inflammation. The inflammatory response peaked between weeks 4 to 8 in musculoskeletal tissues, between weeks 5 and 8 in the median nerve, and partially resolved between weeks 8 and 12 of task performance (Al-Shatti et al., 2005; Barbe et al., 2003; Barbe et al., 2008; Barr et al., 2003; Clark et al., 2003). The data in this paper confirm these previous findings of inflammation in musculotendinous tissues with performance of a moderately demanding task, as well as the correlation of peripheral tissue inflammation with declines in grip strength (Barbe et al., 2008; Elliott et al., 2009b).
However, this current study extends our previous findings in rats performing a MRNF task regarding the declines in motor performance (reduced reach rate and grip strength; Barbe et al., 2003; Clark et al., 2003; Barbe et al., 2008) and a change in the movement pattern used to retrieve a food pellet (raking) (Barbe et al., 2003). We now show new findings of a reduced success rate that correlates with daily exposure, a reduced ability to perform a grooming task, and a loss of grasp control (increased movement reversals, reach and grasp phase times) with performance of the repetitive target task, as well as a direct relationship between these changes and peripheral inflammation and central neural reorganization. What was previously unknown was if peripheral inflammation was primarily responsible for the movement performance deficits that emerge in these rats over time (Barbe and Barr, 2006) or whether cortical degradation was responsible for the movement defects (Byl et al 1997). Based on the findings in this study, it is clear that both sensorimotor cortical reorganization and peripheral inflammation contribute to movement performance declines and movement pattern changes in the progression of the repetitive motion injury. While the proportional contribution of each of these pathophysiological mechanisms remains unknown, these findings serve to focus future experiments designed to examine such a question. These studies are currently underway in our laboratories.
In the primary somatosensory cortex, the repetitive behavioral task resulted mainly in an enlargement of cutaneous RFs across digits (glabrous RFs), increasing their overlap, and across both dorsal and ventral surfaces (hairy (dorsal) and glabrous RFs), an increase in the proportion of cortical sites responding to both forepaw cutaneous stimulation and nail movements in rats. We also observed alterations of S1 neuronal properties and a disruption of the topographic organization of the cutaneous forepaw representation. These data confirm and extend those found in primates in which repetitive, rewarded hand grasp led to a de-differentiation of the finger 3b maps, characterized by enlarged, overlapping RFs, the emergence of multidigit and hairy-glabrous RFs, as well as abnormal somatosensory maps in the thalamus (Byl et al., 1996, 1997; Blake et al., 2002). In previous studies, it has also been shown that abnormal sensorimotor experience (disuse) can result in a degradation of both the topographic organization of somatosensory maps and S1 neuronal properties (Coq and Xerri, 1999; Coq et al., 2008). A concordant degradation of the S1 forepaw map features has been correlated with poor tactile discrimination performance (Xerri et al., 2005). It is now well established that S1 map organization and neuronal properties are correlated with sensorimotor and tactile performances (Duncan and Boynton, 2007; Bensmaia, 2008; Reed et al., 2008). The deterioration of the S1 map features and neuronal properties found in the present study would likely result in ambiguous interpretation of tactile cues and undoubtedly contributed to a decline in grasp control, ultimately resulting in failed and repeated grasp attempts (i.e. increased movement reversals), as well as increased reach and grasp times. The “raking” reach pattern also includes poor forepaw and digit closure and, thus, poor grasp control changes that are reminiscent of focal dystonia as described by Byl et al. (1996, 1997) in an owl monkey model. In fact, in this current study the enlargement of RFs and the emergence of large RFs encompassing the forepaw and forearm correlated with a reduction in successful reaches, an increase in the inefficient raking food retrieval pattern, and an increase in reach time, thus supporting our hypothesis that ambiguous interpretation of tactile cues results in reduced motor performance, as found in the monkey model of focal hand dystonia (Byl et al., 1996, 1997; Blake et al., 2002).
In the motor cortex, the repetitive behavioral task induced an increase in the size of the overall forelimb movement map, task-specific enlargement of multi-joint, elbow-wrist, digit and arm-digit movement representations, as well as reduced amounts of current to evoke digit movements. To a certain extent, the motor cortical reorganization induced by the prolonged performance of repetitive reaching in our model seems more adaptive than deleterious. Several studies examined the effects of motor repetition, related to any motor training experience, on the M1 map organization. Extensive repetition of digit movements without motor learning in primate or unskilled pellet reaching in rats did not significantly alter the M1 forelimb map, while motor skill learning was associated with an expansion of the representation of distal forelimb movements (Kleim et al., 1998; Plautz et al., 2000). The M1 skill-related expansion has been shown to depend upon functional synaptogenesis (Kleim et al., 2002, 2004), long term potentiation (Rioult-Pedotti et al., 2000), excitation-inhibition balance (see Teskey et al., 2008 for a review), synchronous firing (Schieber, 2002), tuning, and signal-to-noise changes of M1 cells (Kargo and Nitz, 2003, 2004). In rats that reached for bundles of pasta strands, skilled reaching with targeting and grasping components requires coordinated forelimb joint movements (Remple et al., 2001). We also observed increases in elbow-wrist and in digit representations, and percent success in our model, changes consistent with increased skill.
Interestingly, as raking increased, so did motor cortical representations for multi-joint movements. This finding in conjunction with the findings for the somatosensory cortical map changes indicates that, with ambiguous sensory input, coordinated movement representations also degrade. Also, peripheral tissue inflammatory changes correlated strongly with expansion of movement representations of the elbow, and with decreased amount of current needed to evoke wrist, elbow-wrist and arm-digit movements. These latter findings suggest that repetitive reaching movements performed over time that are capable of causing tissue injury and inflammation may also reduce movement coordination through reorganization of the somatosensory and motor cortices. The loss of such skill and efficiency would contribute to further increases in movement-induced tissue damage, thereby contributing to a vicious cycle leading toward long term susceptibility to injury and disability, as we have previously hypothesized (Barr and Barbe, 2002; Barbe et al., 2006).
With respect to functional strength, grip strength decreased by the end of the repetitive behavioral task. The correlation results indicate that pro-inflammatory cytokines in forearm muscles and tendons at even the moderate levels observed in this study contribute to the decline in grip strength. Although the inflammatory response reported here is modest, we have previously shown that repetitive motion results in widespread increases of activated macrophages and several pro-inflammatory cytokines in most nerve and musculoskeletal tissues throughout the preferred reach forelimb (Clark et al., 2003; 2004; Barbe et al., 2003; Elliott et al., 2008, 2009a). We hypothesize that it is this widespread nature of the inflammatory response that contributes to our observed motor declines. Studies by Kehl et al. (2000, 2003) also suggest that reduced grip strength is a measure of inflammation-induced muscle hyperalgesia. On the other hand, we have previously shown with our model that fibrotic compression of the median nerve also contributes to declines in grip strength (Clark et al. 2003, 2004). This latter hypothesis is substantiated by a recent study using a pinching task in primates in which performance declined with increased force level and pinch hold time, findings that coincided with a diagnosis of carpal tunnel syndrome (Sommerich et al., 2007). However, poor sensorimotor control may also contribute to declines in strength (Ballermann et al., 2001). Thus, the cortical contribution to this finding cannot be ruled out. Cortical contribution is also supported by our findings of reduced forearm agility (increased reach and grasp phase times, increased movement reversals and reduced forearm grooming function) by week 8 of task performance, changes accompanied by disrupted forepaw representation in the somatosensory cortex and increased representation of multi-joint movements in the motor cortex. In other studies, we also demonstrated the interdependency between musculoskeletal tissue histopathologies and sensorimotor cortex reorganization in the development of abnormal locomotion in rats after disuse during maturation (Strata et al., 2004; Coq et al., 2008). Thus, it is likely that each contribute to motor behavior declines (peripheral tissue inflammation, nerve compression, and maladaptive sensorimotor cortex reorganization). To repeat from above, the proportional contribution of each of these pathophysiological mechanisms remains unknown, and should be the focus of future studies.
The present study contributes strong evidence in support of the learning hypothesis not only for the etiology of repetitive strain disorders but also as a sound theoretical basis for treatment. Cortical somatosensory de-differentiation and motor map reorganization are mechanisms of movement dysfunction that are rarely considered in the examination and treatment of patients with repetitive motion disorders, particularly when there are other signs of peripheral tissue inflammation. Yet, as we have shown here and in conjunction with our previous studies in this model, both peripheral tissue inflammation and cortical maladaptative plasticity are likely to coexist in repetitive motion disorders. The model utilized in this study can be used in future studies as a focus for answering lingering questions regarding the complex pathomechanisms underlying repetitive motion injuries. Further, any reasoned treatment approach should be based upon a more complete understanding of the inter-relationships between the early onset of tissue inflammation, its potential role in driving central neuroplasticity, and the partitioning of the effects of central and peripheral mechanism in causing behavioral declines.
One approach to address the role of inflammation is to antagonize the peripheral immune response and to quantify any resultant changes in cortical reorganization and motor behavior. In recent studies from the Barbe and Barr laboratory, the effectiveness of various anti-inflammatory drugs and non-pharmaceutical interventions designed to reduce inflammation have been explored. We have found not all common anti-inflammatory treatments, e.g. ibuprofen, even at doses known to reduce macrophage influx, completely attenuate peripheral inflammatory responses and associated behavioral declines (Barbe et al, submitted). Therefore, we are currently exploring other pharmaceutical interventions for this purpose. In the mean time, clinicians should not overlook the possibility that motor skill retraining may have to be included in treatment plans in order to regain function and to prevent further tissue damage when managing patients have chronic repetitive motion injury. It also will be important to study the effects of good biomechanics, frequent breaks and strategic movement patterns both in terms of preventing inflammation as well as minimizing cortical de-differentiation.
In conclusion, the present study provides strong evidence for the joint contribution of peripheral tissue inflammation and cortical maladaptative somatosensory and motor plasticity in the pathogenesis of chronic repetitive strain injuries. The findings not only support the learning hypothesis for the etiology of repetitive motion disorders, but the findings also provide a sound theoretical basis for designing effective preventive and restorative strategies. Translating the findings to clinical practice should alert clinicians to address peripheral inflammation as well as cortical somatosensory and cortical motor function in the clinical examination of patients with repetitive strain injury. The animal model utilized in this study provides a strong foundation to continue research regarding the pathogenesis, prevention and remediation of impairments and disabilities associated with repetitive motion injuries.
Acknowledgements
The project described was supported by Grant Number OH 03970 from CDC-NIOSH to MFB and AR051212 from NIAMS to AEB, the Sandler Foundation, NIH Grant NS-10414, Centre National de la Recherche Scientifique (CNRS, in destruction process), Ministère de l’Enseignement Supérieur et de la Recherche, Fondation NRJ – Institut de France to JOC and MR. FS was partly supported by a HFSP long term fellowship LT 00743/1998-B and partly by personal fundings.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jacques-Olivier Coq, UMR 6149 Neurobiologie Intégrative et Adaptative, CNRS - Aix-Marseille Université, Pôle 3C, Case B, 3 Place Victor Hugo, 13331 Marseille Cedex 03, France.
Ann E Barr, Department of Physical Therapy, Jefferson College of Health Professions, Thomas Jefferson University, 130 South 9th Street, Suite 830, Philadelphia, PA 19107, USA.
Fabrizio Strata, Department of Neuroscience, Physiology Section, Medical School, University of Parma, Via Volturno 39/E, 43100 Parma, Italy.
Michael Russier, UMR 6149 Neurobiologie Intégrative et Adaptative, CNRS - Aix-Marseille Université, Pôle 3C, Case B, 3 Place Victor Hugo, 13331 Marseille Cedex 03, France.
David M Kietrys, University of Medicine and Dentistry of New Jersey, School of Health Related Professions and Rutgers, 40 Laurel Road, University Educational Center, Suite 2105, Stratford, NJ , 08084, USA.
Michael M Merzenich, Keck Center for Integrative Neuroscience, University of California San Francisco, 513 Parnassus Avenue, San Francisco, California 94143-0732, USA.
Nancy N Byl, Department of Physical Therapy and Rehabilitation Science, School of Medicine, 1318 7th Ave. Box 0736, University of California San Francisco, San Francisco, CA 94143-0736, USA.
Mary F Barbe, Department of Physical Therapy, College of Health Professions; Department of Anatomy and Cell Biology, Temple Medical School; Temple University, 3307 North Broad Street, Philadelphia, PA, 19140, USA.
References
- Al-Shatti T, Barr AE, Safadi FF, Amin M, Barbe MF. Increase in inflammatory cytokines in median nerves in a rat model of repetitive motion injury. J. Neuroimmunol. 2005;167:13–22. doi: 10.1016/j.jneuroim.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballermann M, McKenna J, Whishaw IQ. A grasp-related deficit in tactile discrimination following dorsal column lesion in the rat. Brain Res. Bull. 2001;54:237–242. doi: 10.1016/s0361-9230(01)00431-2. [DOI] [PubMed] [Google Scholar]
- Barbe MF, Barr AE. Inflammation and the pathophysiology of work-related musculoskeletal disorders. Brain Behav. Immun. 2006;20:423–429. doi: 10.1016/j.bbi.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe MF, Barr AE, Gorzelany I, Amin M, Gaughan JP, Safadi FF. Chronic repetitive reaching and grasping results in decreased motor performance and widespread tissue responses in a rat model of MSD. J. Orthop. Res. 2003;21:167–176. doi: 10.1016/S0736-0266(02)00086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe MF, Elliott MB, Abdelmagid SM, Amin M, Popoff SN, Safadi FF, Barr AE. Serum and tissue cytokines and chemokines increase with repetitive upper extremity tasks. J. Orthop. Res. 2008;26:1320–1326. doi: 10.1002/jor.20674. [DOI] [PubMed] [Google Scholar]
- Barr AE, Barbe MF. Pathophysiological Tissue Changes Associated with Repetitive Movement: A Review of the Evidence. Phys. Ther. 2002;82:173–187. doi: 10.1093/ptj/82.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AE, Safadi FF, Gorzelany I, Amin M, Popoff SN, Barbe MF. Repetitive, negligible force reaching in rats induces pathological overloading of upper extremity bones. J. Bone Miner. Res. 2003;18:2023–2032. doi: 10.1359/jbmr.2003.18.11.2023. [DOI] [PubMed] [Google Scholar]
- Blake DT, Byl NN, Cheung S, Bedenbaugh P, Nagarajan S, Lamb M, Merzenich M. Sensory representation abnormalities that parallel focal hand dystonia in a primate model. Somatosens. Mot. Res. 2002;19:347–357. doi: 10.1080/0899022021000037827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DT, Strata F, Kempter R, Merzenich MM. Experience-dependent plasticity in S1 caused by noncoincident inputs. J. Neurophysiol. 2005;94:2239–2250. doi: 10.1152/jn.00172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensmaia SJ. Tactile intensity and population codes. Behav. Brain Res. 2008;190:165–173. doi: 10.1016/j.bbr.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byl NN, Merzenich MM, Cheung S, Bedenbaugh P, Nagarajan SS, Jenkins WM. A primate model for studying focal dystonia and repetitive strain injury: effects on the primary somatosensory cortex. Phys. Ther. 1997;77:269–284. doi: 10.1093/ptj/77.3.269. [DOI] [PubMed] [Google Scholar]
- Byl NN, McKenzie A. Treatment effectiveness for patients with a history of repetitive hand use and focal hand dystonia: a planned, prospective follow-up study. J. Hand Ther. 2000;13:289–301. doi: 10.1016/s0894-1130(00)80021-6. [DOI] [PubMed] [Google Scholar]
- Byl NN, Mckenzie A, Nagarajan SS. Differences in somatosensory hand organization in a healthy flutist and a flutist with focal hand dystonia: a case report. J. Hand Ther. 2000;13:302–309. doi: 10.1016/s0894-1130(00)80022-8. [DOI] [PubMed] [Google Scholar]
- Byl NN, Melnick M. The neural consequences of repetition: clinical implications of a learning hypothesis. J. Hand Ther. 1997;10:160–174. doi: 10.1016/s0894-1130(97)80070-1. [DOI] [PubMed] [Google Scholar]
- Byl NN, Merzenich MM, Jenkins WM. A primate genesis model of focal dystonia and repetitive strain injury: I. Learning-induced dedifferentiation of the representation of the hand in the primary somatosensory cortex in adult monkeys. Neurology. 1996;47:508–520. doi: 10.1212/wnl.47.2.508. [DOI] [PubMed] [Google Scholar]
- Byl NN, Nagajaran S, McKenzie AL. Effect of sensory discrimination training on structure and function in patients with focal hand dystonia: a case series. Arch. Phys. Med. Rehabil. 2003;84:1505–1514. doi: 10.1016/s0003-9993(03)00276-4. [DOI] [PubMed] [Google Scholar]
- Byl NN, Nagarajan SS, Merzenich MM, Roberts T, McKenzie A. Correlation of clinical neuromusculoskeletal central somatosensory performance: variability in controls and patients with severe and mild focal hand dystonia. Neural Plast. 2002;9:177–203. doi: 10.1155/NP.2002.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candia V, Wienbruch C, Elbert T, Rockstroh B, Ray W. Effective behavioral treatment of focal hand dystonia in musicians alters somatosensory cortical organization. Proc. Nat. Acad. Sci. USA. 2003;100:7942–7946. doi: 10.1073/pnas.1231193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BD, Al-Shatti TA, Barr AE, Amin M, Barbe MF. Performance of a highrepetition, high-force task induces carpal tunnel syndrome in rats. J. Orthop. Sports Phys. Ther. 2004;34:244–253. doi: 10.2519/jospt.2004.34.5.244. [DOI] [PubMed] [Google Scholar]
- Clark BD, Barr AE, Safadi FF, Beitman L, Al-Shatti T, Amin M, Gaughan JP, Barbe MF. Median nerve trauma in a rat model of work-related musculoskeletal disorder. J. Neurotrauma. 2003;20:681–695. doi: 10.1089/089771503322144590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SA, Allard T, Jenkins WM, Merzenich MM. Receptive fields in the bodysurface map in adult cortex defined by temporally correlated inputs. Nature. 1988;332:444–445. doi: 10.1038/332444a0. [DOI] [PubMed] [Google Scholar]
- Coq JO, Xerri C. Environmental enrichment alters organizational features of the forepaw representation in the primary somatosensory cortex of adult rats. Exp. Brain Res. 1998;121:191–204. doi: 10.1007/s002210050452. [DOI] [PubMed] [Google Scholar]
- Coq JO, Xerri C. Tactile impoverishment and sensory-motor restriction deteriorate the forepaw cutaneous map in the primary somatosensory cortex of adult rats. Exp. Brain Res. 1999;129:518–531. doi: 10.1007/s002210050922. [DOI] [PubMed] [Google Scholar]
- Coq JO, Strata F, Russier M, Safadi FF, Merzenich MM, Byl NN, Barbe MF. Impact of neonatal asphyxia and hind limb immobilization on musculoskeletal tissues and S1 map organization: implications for cerebral palsy. Exp. Neurol. 2008;210:95–108. doi: 10.1016/j.expneurol.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Duncan RO, Boynton GM. Tactile hyperacuity thresholds correlate with finger maps in primary somatosensory cortex (S1) Cereb. Cortex. 2007;17:2878–2891. doi: 10.1093/cercor/bhm015. [DOI] [PubMed] [Google Scholar]
- Elliott MB, Barr AE, Kietrys DM, Al-Shatti T, Amin M, Barbe MF. Peripheral neuritis and increased spinal cord neurochemicals are induced in a model of repetitive motion injury with low force and repetition exposure. Brain Res. 2008;1218:103–113. doi: 10.1016/j.brainres.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M, Barr AE, Barbe MF. Spinal Substance P and neurokinin-1 increase with high repetition reaching. 2009b;454(1):33–37. doi: 10.1016/j.neulet.2009.01.037. 2009. [DOI] [PubMed] [Google Scholar]
- Elliott MB, Barr AE, Clark BD, Amin M, Amin S, Barbe MF. High force reaching task induces widespread inflammation, increased spinal cord neurochemicals and neuropathic pain. Neuroscience. 2009a;158:922–931. doi: 10.1016/j.neuroscience.2008.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MB, Barr AE, Kietrys DM, Amin M, Barbe MF. Peripheral neuritis and spinal cord neurochemicals are induced in a model of repetitive motion injury with minimal force and repetition exposure. Brain Res. 2008;1218:103–113. doi: 10.1016/j.brainres.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargo WJ, Nitz DA. Early skill learning is expressed through selection and tuning of cortically represented muscle synergies. J. Neurosci. 2003;23:11255–11269. doi: 10.1523/JNEUROSCI.23-35-11255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargo WJ, Nitz DA. Improvements in the signal-to-noise ratio of motor cortex cells distinguish early versus late phases of motor skill learning. J. Neurosci. 2004;24:5560–5569. doi: 10.1523/JNEUROSCI.0562-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl LJ, Hamamoto DT, Wacnik PW, Croft DL, Norsted BD, Wilcox GL, Simone DA. A cannabinoid agonist differentially attenuates deep tissue hyperalgesia in animal models of cancer and inflammatory muscle pain. Pain. 2003;103:175–186. doi: 10.1016/s0304-3959(02)00450-5. [DOI] [PubMed] [Google Scholar]
- Kehl LJ, Trempe TM, Hargreaves KM. A new animal model for assessing mechanisms and management of muscle hyperalgesia. Pain. 2000;85:333–343. doi: 10.1016/S0304-3959(99)00282-1. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J. Neurophysiol. 1998;80:3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Cooper NR, Hogg TM, Reidel CN, Remple MS, Nudo RJ. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol. Learn. Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J. Neurosci. 2004;24:628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie AL, Nagarajan SS, Roberts TP, Merzenich MM, Byl NN. Somatosensory representation of the digits and clinical performance in patients with focal hand dystonia. Am. J. Phys. Med. Rehabil. 2003;82:737–749. doi: 10.1097/01.PHM.0000087458.32122.14. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, Terreberry RR. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 1996;396:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Jenkins WM, Merzenich MM. Repetitive microstimulation alters the cortical representation of movements in adult rats. Somatosens. Mot. Res. 1990;7:463–483. doi: 10.3109/08990229009144720. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J. Neurosci. 1996;16(2):785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BE, Merzenich MM. MAP: a Macintosh program for generating categorical maps applied to cortical mapping. J. Neurosci. Methods. 1995;57:133–144. doi: 10.1016/0165-0270(94)00103-n. [DOI] [PubMed] [Google Scholar]
- Reed JL, Pouget P, Qi HX, Zhou Z, Bernard MR, Burish MJ, Haitas J, Bonds AB, Kaas JH. Widespread spatial integration in primary somatosensory cortex. Proc. Natl. Acad. Sci. USA. 2008;105:10233–10237. doi: 10.1073/pnas.0803800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remple MS, Bruneau RM, VandenBerg PM, Goertzen C, Kleim JA. Sensitivity of cortical movement representations to motor experience: evidence that skill learning but not strength training induces cortical reorganization. Behav. Brain Res. 2001;123:133–141. doi: 10.1016/s0166-4328(01)00199-1. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Training and synchrony in the motor system. J. Neurosci. 2002;22:5277–5281. doi: 10.1523/JNEUROSCI.22-13-05277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerich CM, Lavender SA, Buford JA, Banks JJ, Korkmaz SV, Pease WS. Towards development of a nonhuman primate model of carpal tunnel syndrome: performance of a voluntary repetitive pinching task induces median mononeuropathy in Macaca fascicularis. J. Orthop. Res. 2007;25:713–724. doi: 10.1002/jor.20363. [DOI] [PubMed] [Google Scholar]
- Strata F, Coq JO, Byl N, Merzenich MM. Effects of sensorimotor restriction and anoxia on gait and motor cortex organization: implications for a rodent model of cerebral palsy. Neuroscience. 2004;129:141–156. doi: 10.1016/j.neuroscience.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Teskey GC, Monfils MH, Flynn C, Young NA, van Rooyen F, Henry LC, Ozen LJ, Henderson AK, Reid AY, Brown AR. Motor maps, seizures, and behaviour. Can. J. Exp. Psychol. 2008;62:132–139. doi: 10.1037/1196-1961.62.2.132. [DOI] [PubMed] [Google Scholar]
- Topp KS, Byl NN. Movement dysfunction following repetitive hand opening and closing: anatomical analysis in owl monkeys. Movement Disorders. 1999;14:295–306. doi: 10.1002/1531-8257(199903)14:2<295::aid-mds1015>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Wang X, Merzenich MM, Sameshima K, Jenkins WM. Remodeling of hand representation in adult cortex determined by timing of tactile stimulation. Nature. 1995;378:71–75. doi: 10.1038/378071a0. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM. The structure of skilled forelimb reaching in the rat: a proximally driven movement with a single distal rotatory component. Behav. Brain Res. 1990;41:49–59. doi: 10.1016/0166-4328(90)90053-h. [DOI] [PubMed] [Google Scholar]
- Xerri C, Bourgeon S, Coq JO. Perceptual context-dependent remodelling of the forepaw map in the SI cortex of rats trained on tactile discrimination. Behav. Brain Res. 2005;162:207–221. doi: 10.1016/j.bbr.2005.03.003. [DOI] [PubMed] [Google Scholar]