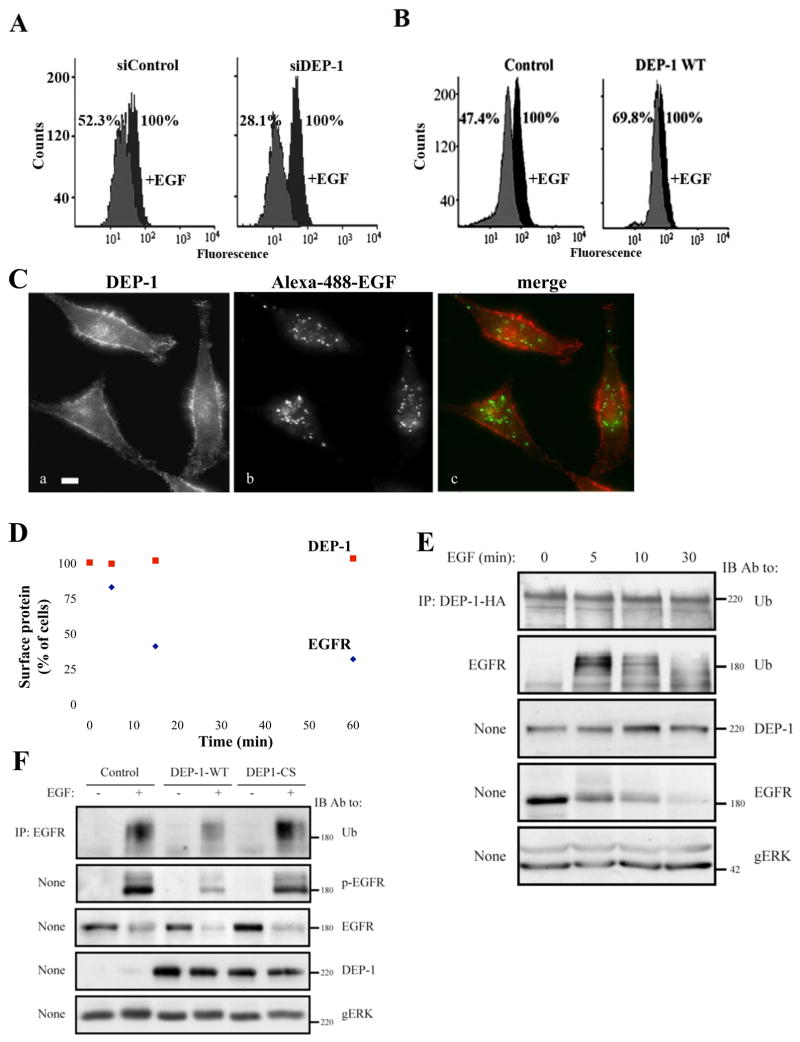
Figure 6. DEP-1 Inhibits EGFR Internalization and Ubiquitinylation and Remains on the Cell Surface After EGFR is Internalized.
(A) HeLa cells were transfected with the indicated siRNA oligonucleotides, and 32h later they were starved for 16h and un-treated or treated with EGF (20ng/ml; 10 min). Thereafter, surface localized EGFR was quantified by flow cytometry. Numbers represent percents of initial cell surface EGFR.
(B) HeLa cells transiently expressing HA-DEP-1 (or a control vector) were assayed as in A. One representative experiment (n=2) is shown.
(C) HeLa cells were stimulated with a fluorescently labeled EGF (FITC-EGF; 20ng/ml) for 30 min at 22°C, fixed and analyzed by immunofluorescence using an antibody to DEP-1.
(D) HeLa cells were serum-starved for 16h and stimulated with EGF (20ng/ml) for the indicated time intervals. Thereafter, cells were surface labeled with antibodies to EGFR and DEP-1. The remaining surface fraction of each protein was quantified by flow cytometry.
(E) HeLa cells expressing DEP-1-HA were stimulated with EGF (20ng/ml) for the indicated time intervals. EGFR and the ectopically expressed DEP-1 were immunoprecipitated from cell lysates and subjected to immunoblotting.
(F) HeLa cells were treated as in E and stimulated with EGF (20ng/ml; 5 min). EGFR was analyzed by immunoblotting, either directly of after immunoprecipitation. EGFR phosphorylation was detected using an anti-phosphotyrosine antibody.