Figure 3.
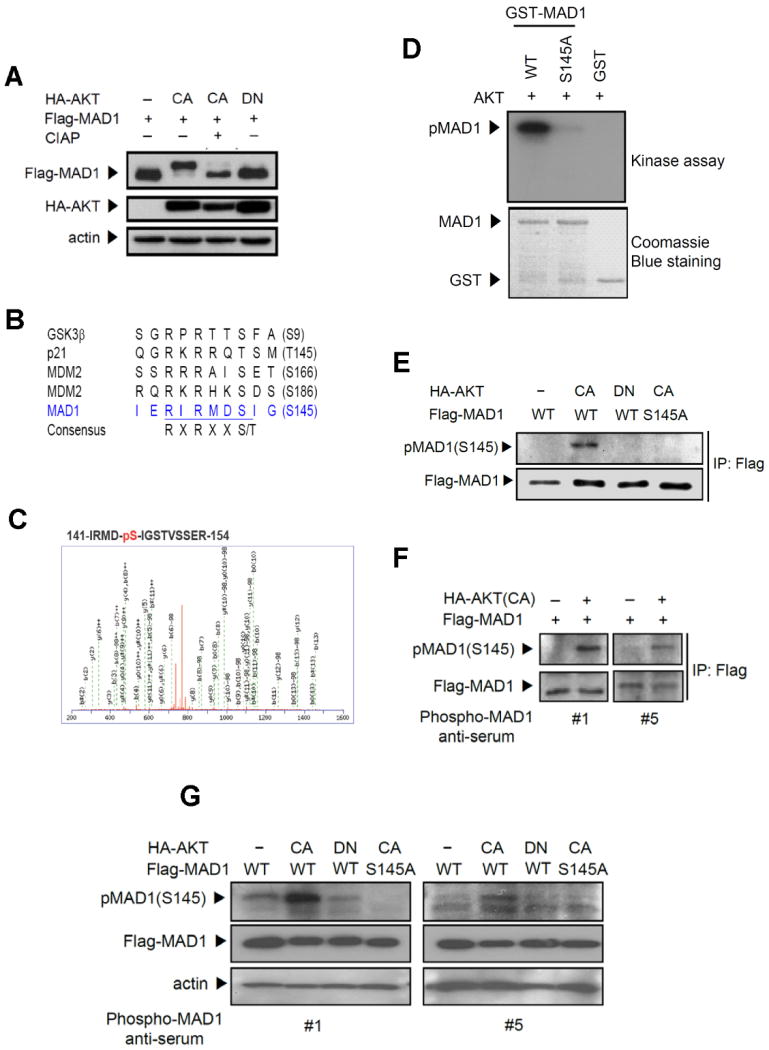
AKT phosphorylates MAD1 both in vitro and in vivo. (A) AKT induced a mobility shift of MAD1, and calf intestine alkaline phosphatase (CIAP) reversed it. Lysates from 293 cells transfected with HA-AKT and Flag-MAD1 were treated with CIAP and subjected to immunoblotting. (B) MAD1 has a putative AKT phosphorylation site at Ser145 (RIRMDS145). R, arginine; S, serine; T, threonine; X, any amino acid. (C) Mass spectrometric analysis of AKT-mediated MAD1 phosphorylation site on GST-MAD1 at Ser145. (D) MAD1 phosphorylation site Ser145 was identified by in vitro kinase assays in which recombinant AKT was incubated with GST-MAD1 and mutant GST-MAD1 (S145A) fusion proteins. (E) AKT-mediated phosphorylation of MAD1 was detected by phospho-(Ser/Thr) AKT substrate antibody and showed in vivo phosphorylation in the presence of AKT. Lysates from 293 cells transfected with the indicated AKT and MAD1 were subjected to immunoblotting. (F) Characterization of antibodies to pMAD1 (S145) by immunoprecipitation. Lysates from 293 cells transfected with MAD1 and AKT were immunoprecipitated by anti-Flag antibody and immunoblotting was performed using phospho-MAD1 anti-serum #1 and #5. (G) MAD1 Ser145 in vivo phosphorylation was detected by direct immunoblotting. Lysates were prepared as in (E).
