Figure 4.
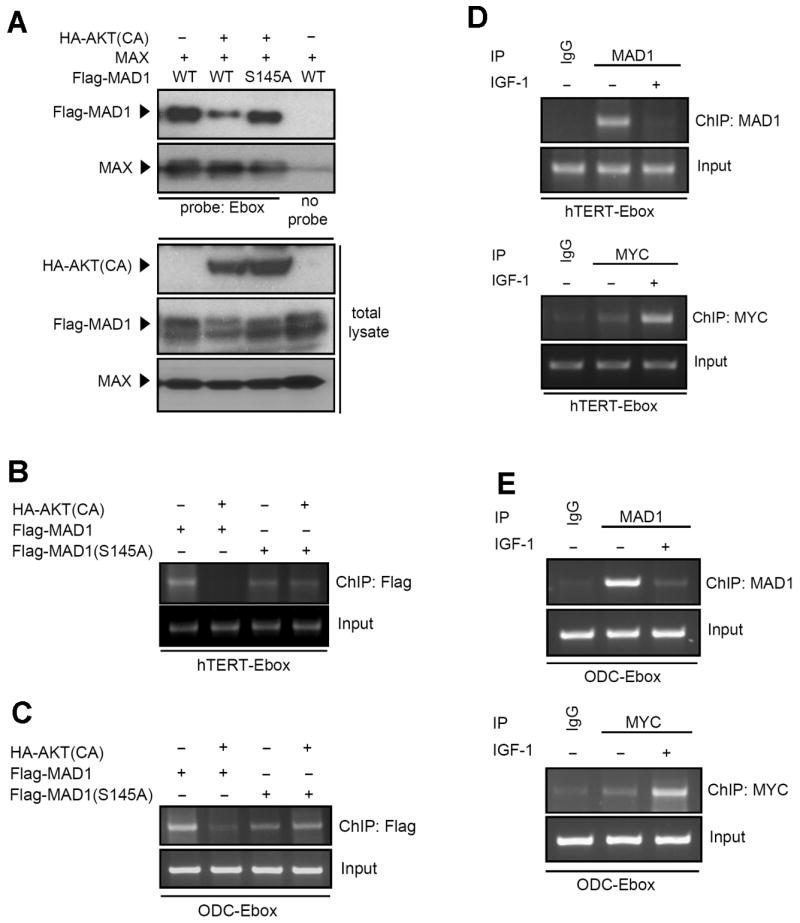
Phosphorylation of MAD1 affects its target DNA binding. (A) DNA binding affinity assay showed that MAD1 phosphorylation affects its target DNA binding. Lysates of 293 cells transfected with designed plasmids were incubated with biotin-conjugated hTERT-Ebox DNA probe and pulled down by streptavidin beads. Probes pulled down proteins were subjected to immunoblotting by anti-Flag, anti-HA, and MAX antibodies. (B) AKT-mediated phosphorylation affects hTERT promoter binding to the MAD1 target gene but not to the MAD1 S145A mutant. Lysates of HeLa cells transfected with the indicated plasmids were subjected to ChIP analysis with anti-Flag antibody. (C) Phosphorylation mediated MAD1 promoter binding alternation was also shown in ChIP assay with anti-Flag antibody and ODC promoter. The experimental setup was the same as for (B). (D) IGF-1 stimulation suppressed MAD1 and induced MYC binding on the hTERT promoter. Lysates of MCF7 cells, treated as indicated, were subjected to the ChIP assay with anti-MAD1 and anti-MYC antibodies. (E) Transcription factors that switched from MAD1 to MYC upon IGF-1 stimulation were also shown in the ODC promoter ChIP assay with anti-MAD1 and anti-MYC antibodies.
